Multiple-parameter profiling of density gradient ultracentrifugation for characterization of empty and full capsid distribution in AAV preparations
Cell & Gene Therapy Insights 2021; 7(2), 161–169
10.18609/cgti.2021.039
Ultracentrifugation (UC) is a well-known technique for fractionating adeno-associated virus (AAV) capsids according to their density, which is mainly a function of their encapsidated DNA mass. Empty capsids represent the lowest density subpopulation. Full capsids represent the highest density subpopulation, sometimes accompanied by partially full capsids of intermediate density. Fractions can be collected after sedimentation for analysis but the practice is laborious and discourages application of multiple monitoring techniques that might provide deeper insights into sample composition. Anion exchange chromatography (AEC) also achieves fractionation of empty and full capsids for many AAV serotypes. The degree of separation varies among serotypes and does not correlate strictly with UC. This is not surprising since separation by AEC is highly influenced by capsid surface charge, which is independent of the amount of DNA packaged within the capsids. Chromatography methods however present a significant analytical advantage in the ease of monitoring the column effluent, including with multiple detectors. UV absorbance at 260 nm and 280 nm permits estimation of empty and full capsid proportions in any given peak. Intrinsic fluorescence enables estimation of relative areas of empty capsid peaks and full capsid peaks. Light scattering does the same and permits the further determination of capsid size and mass. In this report, we merge UC with an HPLC monitoring array to simultaneously analyze dual wavelength UV, intrinsic fluorescence, and light scattering through cesium chloride density gradient strata. Limitations of each monitoring method are discussed. UC results are compared with chromatography profiles to highlight distinction between separation methods. Practical application of results for final product characterization is considered, along with potential to support development of better purification processes.
Introduction
Density gradient ultracentrifugation (DGUC) is a well-known technique for fractionating adeno-associated virus (AAV) capsids according to the amount of encapsidated DNA they contain [1]Ayuso E, Mingozzi, F, Montane, J et al. AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency, Gene Ther. 2010; 17: 503–10.. Empty capsids represent the lowest density subpopulation. Full capsids represent the highest density subpopulation, sometimes accompanied by partially full capsids of intermediate density. Fractions can be collected after sedimentation for analysis [2]Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene. Ther. Met. 2015; 26: 147–57. Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene. Ther. Met. 2015; 26: 147–57. but the practice is laborious and discourages application of multiple monitoring techniques that might provide deeper insights into sample composition. The concept of flowing density gradient-separated bacteriophage fractions through a UV monitor was demonstrated in 1978 and offers further potential for AAV [3]Griffith OM. Rapid density gradient centrifugation using short column techniques. Anal. Biochem. 1978; 90: 435–4. The method known as Analytical Ultra-Centrifugation (AUC) pertains to a different technique that is also applied to AAV [4]Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.. AUC does not exploit density gradients but relies instead on differences in the inherent sedimentation coefficients among sample components.
Anion exchange chromatography (AEX) also achieves fractionation of empty and full capsids for many AAV serotypes [4]Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52., [5]Wang C, Mulagapati S, Chen Z et al. Developing an anion exchange assay for determining empty and full capsid contents in AAV6.2. Mol. Ther. 2019; 15: 257–63.Wang C, Mulagapati S, Chen Z et al. Developing an anion exchange assay for determining empty and full capsid contents in AAV6.2. Mol. Ther. 2019; 15: 257–63., [6]Dickerson R, Argento C, Pieracci J, Bakhshayeshi M. Separating empty and full recombinant Adeno-associated virus particles using isocratic anion exchange chromatography. Biotechnol. J. 2020., [7]Qu, G, Bahr-Davidson, J, Proado, J et al. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion exchange chromatography. J. Virol. Met. 2007; 140: 183–92., [8]Lock M, Alvira M, Wilson JM. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion exchange chromatography. Hum. Gene Ther. Met. 2012; 23., [9]Lock, M, Alvira, M. Scalable purification method for AAV World Patent Application. 2017, WO2017160360A9: https://patents.google.com/patent/WO2017160360A9/enLock, M, Alvira, M. Scalable purification method for AAV World Patent Application. 2017, WO2017160360A9: https://patents.google.com/patent/WO2017160360A9/en, [10]Urabe, M, Xin, K-Q, Obara, Y et al. Removal of empty capsids from type 1 adeno-associated virus vector stocks by anion exchange chromatography potentiates transgene expression. Mol. Ther. 2006; 13: 823–8., [11]Brument N, Morenweiser R, Bioquin V et al. A versatile and scalable two-step ion exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol. Ther. 2002; 6: 678–86., [12]Davidoff AM, Ng CYC, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Met. 2004; 121: 209–15.Davidoff AM, Ng CYC, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Met. 2004; 121: 209–15., [13]Kaludov N, Handelman B, Chiorina JA. Scalable purification of adeno-associated virus type 2, 4, or 5, using ion exchange chromatography. Hum. Gene Ther. 2004; , [14]Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. , [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.. The degree of separation varies among serotypes and does not correlate strictly with DGUC. This is not surprising since separation by AEX is highly influenced by capsid surface charge, which is independent of the amount of DNA packaged within the capsids. Chromatography methods however present a significant analytical advantage in the ease of monitoring the column effluent, including with multiple detectors. Calculating the ratio of UV absorbance at 260 nm to absorbance at 280 nm permits estimation of empty and full capsid proportions in any given peak [4]Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52., [5]Wang C, Mulagapati S, Chen Z et al. Developing an anion exchange assay for determining empty and full capsid contents in AAV6.2. Mol. Ther. 2019; 15: 257–63.Wang C, Mulagapati S, Chen Z et al. Developing an anion exchange assay for determining empty and full capsid contents in AAV6.2. Mol. Ther. 2019; 15: 257–63., [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.. Intrinsic fluorescence enables estimation of relative areas of empty capsid peaks versus full capsid peaks [4]Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52.Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52., [14]Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. , [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.. Light scattering does the same and permits the further determination of capsid size and mass [14]Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. , [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113., [16]McIntosh NL, Berguig GY, Karim OA et al. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021; 11: 3012.McIntosh NL, Berguig GY, Karim OA et al. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021; 11: 3012..
In this report, we present an expanded DGUC method for characterization of empty and full AAV capsid content in cell culture harvests, lysates, and chromatography fractions. The contents of post-DGUC tubes are pumped through an HPLC monitoring array to measure UV absorbance, intrinsic fluorescence, and light scattering across cesium chloride density strata. Conductivity is measured as a surrogate indicator of cesium chloride density. Signal integration produces a multi-parameter DGUC ‘centrifugram’ that corresponds in many respects to the chromatograms produced by chromatography methods. DGUC results are compared with chromatography profiles to highlight distinctions between separation methods. Practical application of results for final product characterization is considered, along with potential to support development of better purification processes.
Materials & methods
AAV8 lysates produced from Sf9/BEV cells were obtained from the University of Nantes, INSERM UMR 1089, Nantes, France. AAV8 was chosen because AEX is documented to separate empty and full AAV8 capsids [9]Lock, M, Alvira, M. Scalable purification method for AAV World Patent Application. 2017, WO2017160360A9: https://patents.google.com/patent/WO2017160360A9/enLock, M, Alvira, M. Scalable purification method for AAV World Patent Application. 2017, WO2017160360A9: https://patents.google.com/patent/WO2017160360A9/en, [12]Davidoff AM, Ng CYC, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Met. 2004; 121: 209–15.Davidoff AM, Ng CYC, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Met. 2004; 121: 209–15., [14]Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. , [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113. and thereby facilitate comparison of empty/full separation by AEC and DGUC. Initial AAV purification was performed by cation exchange chromatography (CEX) on a 1 mL CIMmultus® SO3 monolith (BIA Separations). CEX columns were equilibrated to 50 mM formic acid, 200 mM sodium chloride, 1% sucrose, 0.1% Poloxamer[CV]/min). AEX fractionation of CEX-purified AAV was performed on a CIMmultus® QA monolith (BIA Separations). The column was equilibrated with 50 mM bis-tris-propane, 2 mM magnesium chloride, pH 9.0; eluted with a linear salt gradient to 50 mM bis-tris-propane, 2 mM magnesium chloride, 200 mM sodium chloride, pH 9.0; then cleaned with 2 M sodium chloride plus 1 M sodium hydroxide.
Density gradient fractionation was performed on a Sorvall™ WX 90+ ultracentrifuge (Thermo Scientific) using 11.5 mL polyethylene UltraCrimp® centrifuge tubes (Thermo Scientific) in a T890 fixed-angle rotor. Samples containing about 1E+11 vector genomes (vg) according to ddPCR as described in [17]Dobnik D, Kogovsek P, Jakomin T et al. Accurate quantification and characterization of adeno-associated viral vectors. Front. Microbiol. 2019; 10: 1570. were mixed with concentrated cesium chloride to obtain an AAV sample in 3 M cesium chloride. Empty capsid sample volumes/concentrations were estimated based on the relative size of the empty and full capsid peaks from AEX. Centrifugation was performed at 53,500 RPM for 24 h at room temperature. The tube was then fixed in a stand and pierced near the top with a hypodermic needle (23 gauge, 70 mm, B Braun) extending to bottom-center (Figure 1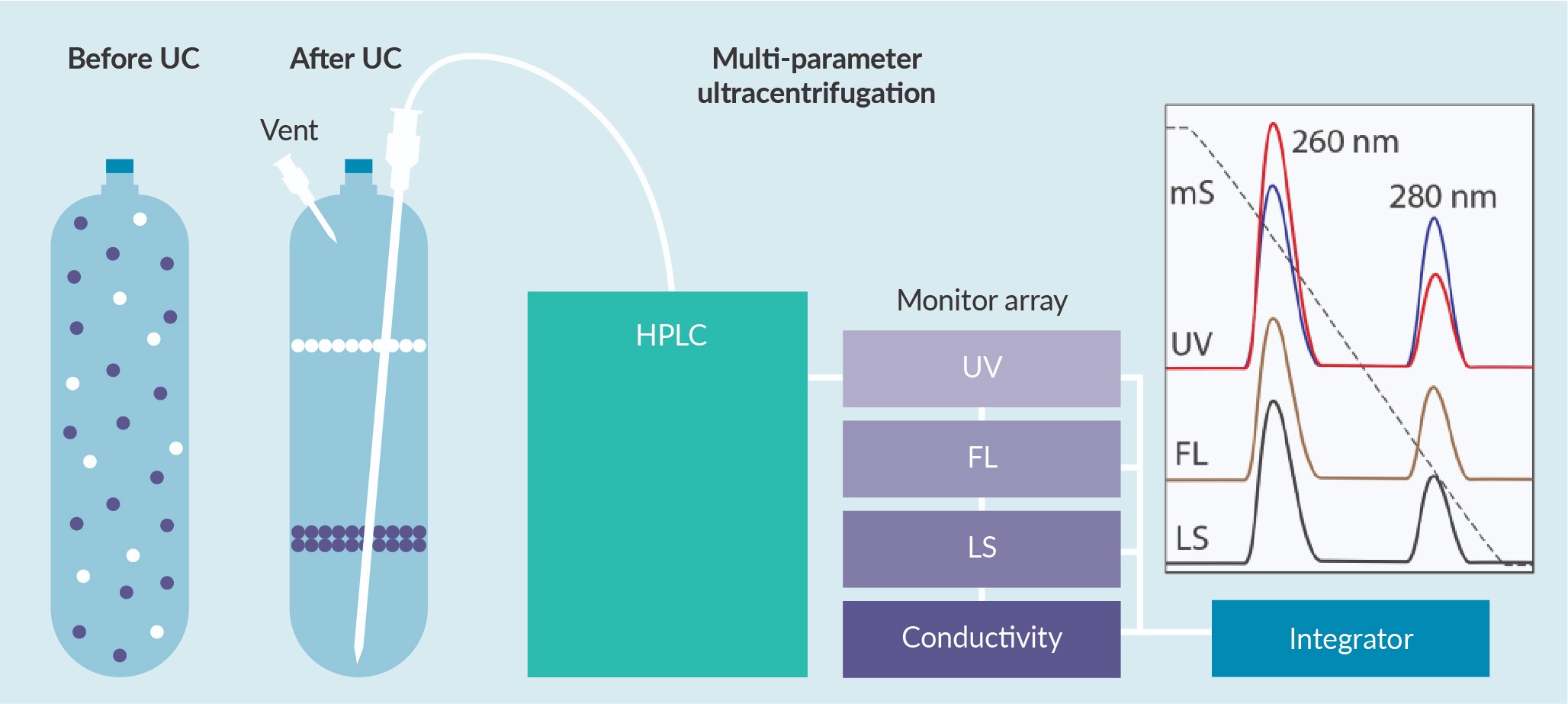
The system was washed with water between samples. The discontinuity of refractive index between the water in the HPLC tubing and cesium chloride in the next sample created heavy signal noise at the beginning of the method. To properly zero the baseline, 1 mL of fluid was passed through the system to equilibrate the monitors to cesium chloride before the system was zeroed and data collection begun.
Results & discussion
Figure 2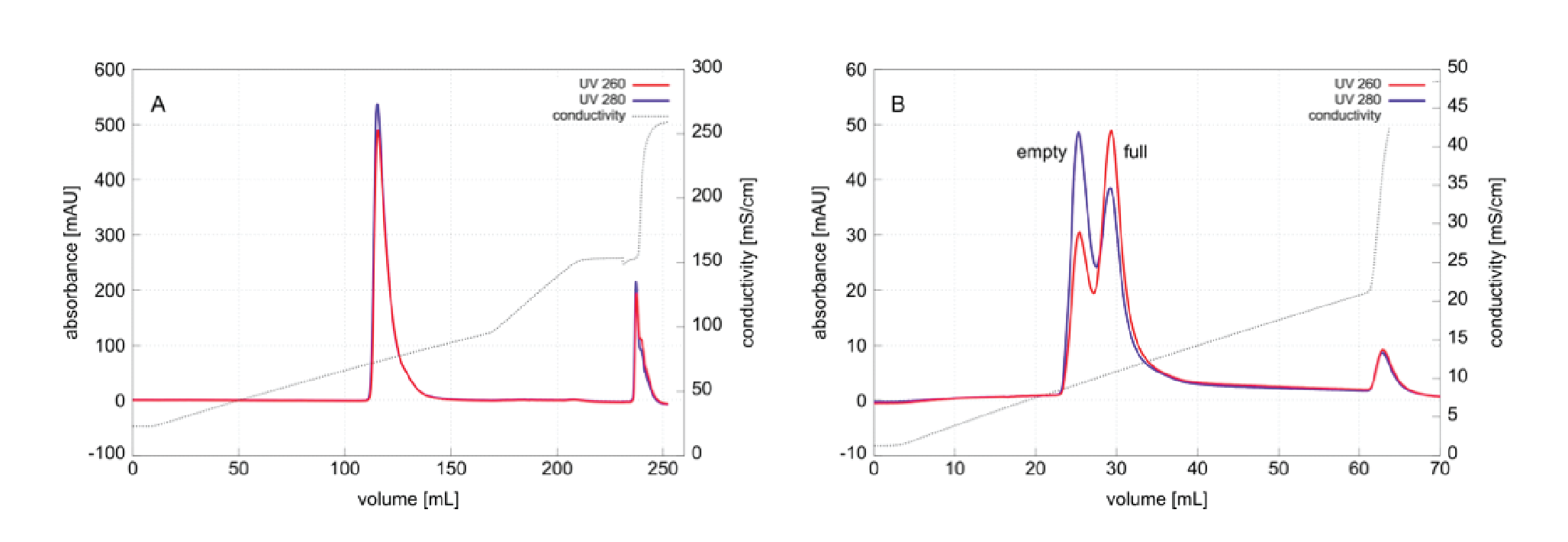
The UV absorbance ratio was unfortunately unable to provide more definition because of the increasing baseline across the profile. This is an artifact created by changes in refractive index across the density gradient, but also note that it affects the 260 nm baseline more than the 280 nm baseline. This complicates calculation of wavelength ratios and ultimately limits sensitivity. Samples containing fewer capsids can be detected by increasing monitor sensitivity but higher sensitivity also increases relative baseline slope. This puts 1E+11 vg close to the lower limit of capsid numbers required for UV monitoring. Baselines for intrinsic fluorescence and light scattering were flat, which means that sensitivity can be increased without compromising measurement accuracy. Present results suggest that running the method with 1E+10 vg or fewer capsids will likely produce useful intrinsic fluorescence and light scattering data.
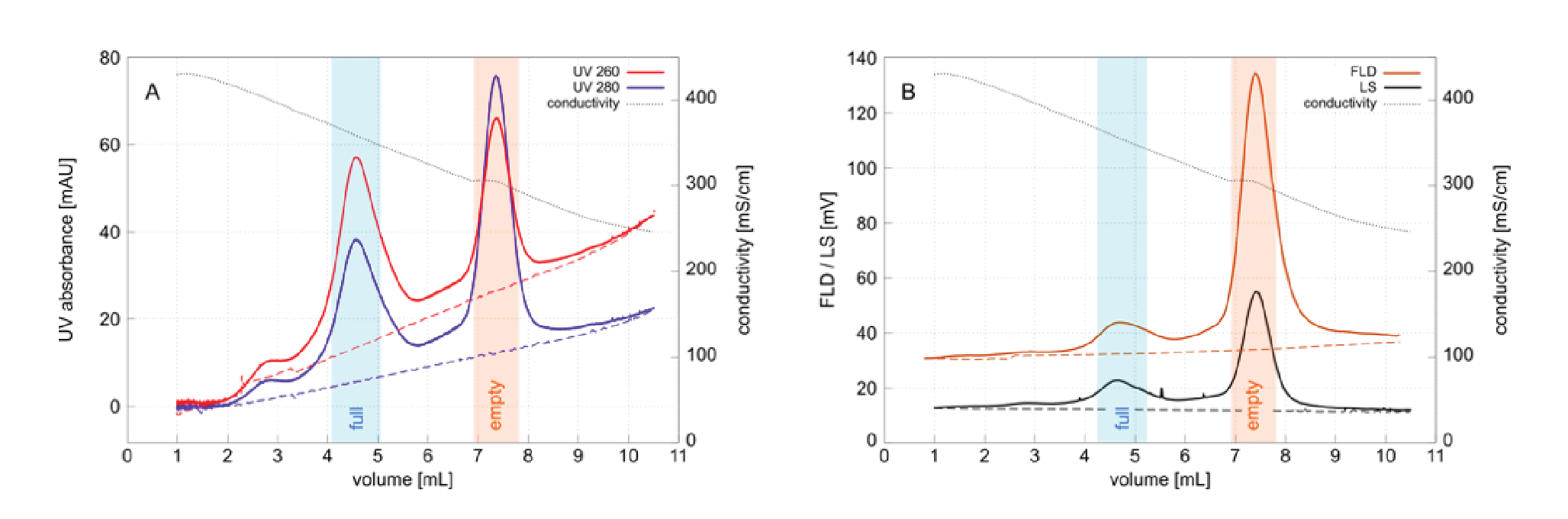
Previously published results suggested the high density peak at 2.75 min might correspond to mispackaged plasmid DNA [2]Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene. Ther. Met. 2015; 26: 147–57. Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene. Ther. Met. 2015; 26: 147–57. . The present results suggest that population may rather represent dis-packaged DNA. The 260/280 ratio of about 1.4 is similar to full capsids but does not explain why they would exhibit higher density. Refractive index dependency of the UV baseline compromises precise estimation of DNA to protein ratio based on UV, but this population is clearly DNA-rich and intrinsic fluorescence confirms the presence of capsid proteins. Light scattering intensity unfortunately does not indicate how large the particles might be. Particle size and particle concentration both contribute to light scattering intensity and knowing one is required to interpret the other [16]McIntosh NL, Berguig GY, Karim OA et al. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021; 11: 3012.McIntosh NL, Berguig GY, Karim OA et al. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021; 11: 3012..
One hypothesis that is consistent with both the published results and the experimental data is that this population may represent aggregated full capsid debris created by exposure to cesium chloride under the high shear stress of ultracentrifugation. AAV capsid instability during DGUC has been noted by others who recommended inclusion of 10 mM magnesium ions to stabilize them [18]Wright JF, Le T, Prado J et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol. Ther. 2005; 12: 171–8., [19]Wright JF, Qu G. Compositions and methods to prevent AAV vector aggregation, United States Patent US20110076744A1, March 3, 2011: https://patents.google.com/patent/US20110076744A1/en . Unpurified harvests and lysates sometimes exhibit a larger proportion of high density material (not shown) that suggests residual chromatin heteroaggregates might contribute to this population. Chromatin heteroaggregates exist as highly condensed structures that range in size up to 400 nm and are known to persist in 2 M sodium chloride [20]Gagnon P, Nian R, Tan L et al. Chromatin-mediated depression of fractionation performance on electronegative multimodal chromatography media, its prevention, and ramifications for purification of IgG, J. Chromatogr. A 2014; 1374: 145–55.Gagnon P, Nian R, Tan L et al. Chromatin-mediated depression of fractionation performance on electronegative multimodal chromatography media, its prevention, and ramifications for purification of IgG, J. Chromatogr. A 2014; 1374: 145–55.. Chromatin cannot be entirely ruled out as a contributor to the high density population in Figure 3 but it seems unlikely to be a major contributor because cation exchange has been shown to remove the majority of it [15]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113.. AEC reduces chromatin levels further [20]Gagnon P, Nian R, Tan L et al. Chromatin-mediated depression of fractionation performance on electronegative multimodal chromatography media, its prevention, and ramifications for purification of IgG, J. Chromatogr. A 2014; 1374: 145–55.Gagnon P, Nian R, Tan L et al. Chromatin-mediated depression of fractionation performance on electronegative multimodal chromatography media, its prevention, and ramifications for purification of IgG, J. Chromatogr. A 2014; 1374: 145–55. yet the high density population is also observed in the full capsid peak from AEC (Figure 4), again pointing to degradation during DGUC.
Figure 4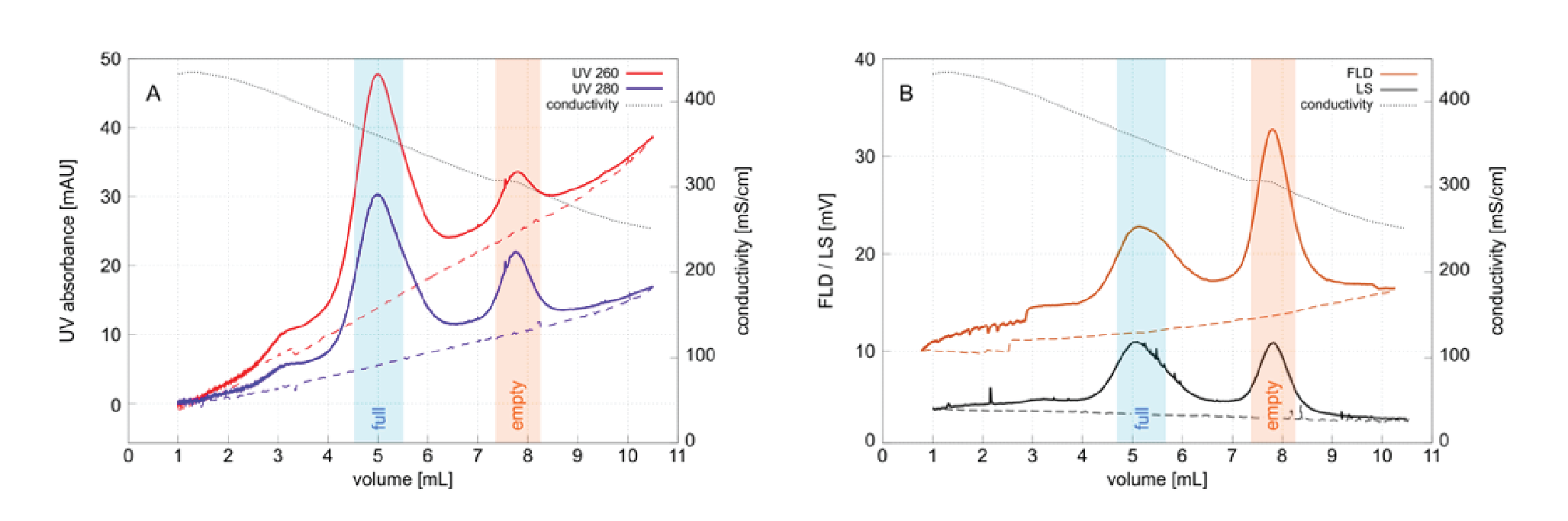
Figure 5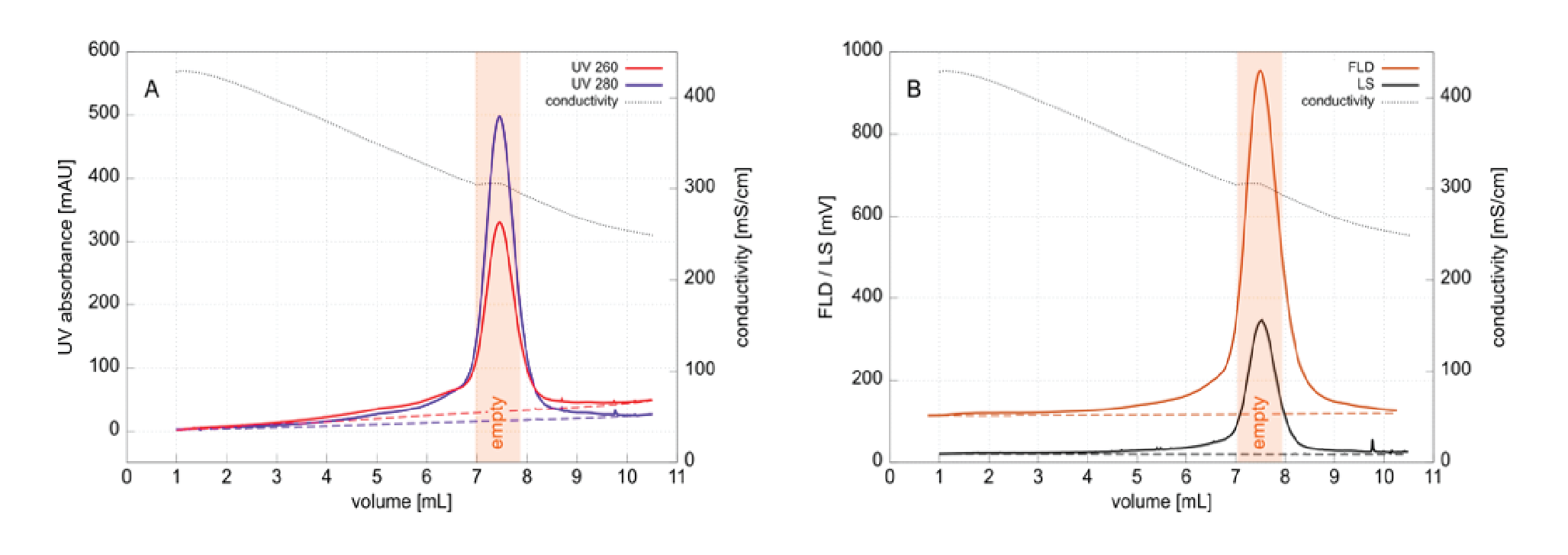
Conclusions
Characterizing DGUC profiles with multiple monitors provides valuable new perspectives for characterization of empty and full capsid distribution in AAV preparations. It is orthogonal to separation of empty and full capsids by AEX and enables more accurate interpretation of AEX chromatograms. This information can be used to better guide development of purification processes. It can also be used to guide development of density gradient formulations that may better conserve capsid stability during DGUC. Further characterization of the technique with capsids from other serotypes, lysates, harvests, chromatography fractions under various conditions and with differing abilities to separate empty and full capsids, all represent important opportunities to determine the full potential of the technique. Its performance with iodixanol and other density gradient media also promises to be interesting.
References
1. Ayuso E, Mingozzi, F, Montane, J et al. AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency, Gene Ther. 2010; 17: 503–10. Crossref
2. Strobel B, Miller FD, Rist W, Lamla T. Comparative analysis of cesium chloride and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum. Gene. Ther. Met. 2015; 26: 147–57. Crossref
3. Griffith OM. Rapid density gradient centrifugation using short column techniques. Anal. Biochem. 1978; 90: 435–43. Crossref
4. Fu X, Chen W-C, Argento C et al. Analytical strategies of quantitation of adeno-associated virus empty capsids to support process development. Hum. Gene Ther. Met. 2019; 30: 144–52. Crossref
5. Wang C, Mulagapati S, Chen Z et al. Developing an anion exchange assay for determining empty and full capsid contents in AAV6.2. Mol. Ther. 2019; 15: 257–63. Crossref
6. Dickerson R, Argento C, Pieracci J, Bakhshayeshi M. Separating empty and full recombinant Adeno-associated virus particles using isocratic anion exchange chromatography. Biotechnol. J. 2020. Crossref
7. Qu, G, Bahr-Davidson, J, Proado, J et al. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion exchange chromatography. J. Virol. Met. 2007; 140: 183–92. Crossref
8. Lock M, Alvira M, Wilson JM. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion exchange chromatography. Hum. Gene Ther. Met. 2012; 23. Crossref
9. Lock, M, Alvira, M. Scalable purification method for AAV9. World Patent Application. 2017, WO2017160360A9: https://patents.google.com/patent/WO2017160360A9/en
10. Urabe, M, Xin, K-Q, Obara, Y et al. Removal of empty capsids from type 1 adeno-associated virus vector stocks by anion exchange chromatography potentiates transgene expression. Mol. Ther. 2006; 13: 823–8. Crossref
11. Brument N, Morenweiser R, Bioquin V et al. A versatile and scalable two-step ion exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol. Ther. 2002; 6: 678–86. Crossref
12. Davidoff AM, Ng CYC, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Met. 2004; 121: 209–15. Crossref
13. Kaludov N, Handelman B, Chiorina JA. Scalable purification of adeno-associated virus type 2, 4, or 5, using ion exchange chromatography. Hum. Gene Ther. 2004; 13. Crossref
14. Gagnon, P, Leskovec, M, Goricar, B, Strancar, A. Streamlining industrial purification of adeno-associated virus. BioProcess Intl. 2020; 18: S14–S20. Crossref
15. Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 17: 113. Crossref
16. McIntosh NL, Berguig GY, Karim OA et al. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021; 11: 3012. Crossref
17. Dobnik D, Kogovsek P, Jakomin T et al. Accurate quantification and characterization of adeno-associated viral vectors. Front. Microbiol. 2019; 10: 1570. Crossref
18. Wright JF, Le T, Prado J et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol. Ther. 2005; 12: 171–8. Crossref
19. Wright JF, Qu G. Compositions and methods to prevent AAV vector aggregation, United States Patent US20110076744A1, March 3, 2011: https://patents.google.com/patent/US20110076744A1/en Crossref
20. Gagnon P, Nian R, Tan L et al. Chromatin-mediated depression of fractionation performance on electronegative multimodal chromatography media, its prevention, and ramifications for purification of IgG, J. Chromatogr. A 2014; 1374: 145–55. Crossref
21. Sommer JM, Smith PH, Parthasarathy S et al. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003; 7: 122–8. Crossref
22. Pierson E, Keifer DZ, Asokan A, Jarrold M. Resolving adeno-associated viral particle diversity with charge detection mass spectrometry. Anal. Chem. 2016; 88: 6718–25. Crossref
23. Han Y, Li D, Chen W, Mu S, Chen Y, Chai J. Impact of refractive index increment on the determination of molecular weight of hyaluronic acid by multi-angle laser light scattering technique. Sci. Rep. 2020; 10: 1858. Crossref
24. Wörner TP, Bennet A, Habka S et al. Adeno-associated virus capsid assembly is divergent and stochastic. bioRxiv 2020. Crossref
25. Bertin M, Maurya S, Arumugam S, Kumar V, Jayadharan GR. Post-translational modifications in capsid proteins of recombinant Adeno-associated virus (AAV) 1-rh19 serotypes. FEBS J. 2019; 286: 4964–81. Crossref
Affiliations
Sebastijan Peljhan
BIA Separations d.o.o.
(part of Sartorius),
Mirce 21, 5270 Ajdovscina, Slovenia
Maja Štokelj
BIA Separations d.o.o.
(part of Sartorius),
Mirce 21, 5270 Ajdovscina, Slovenia
Sara Drmota Prebil
BIA Separations d.o.o.
(part of Sartorius),
Mirce 21, 5270 Ajdovscina, Slovenia
Pete Gagnon
BIA Separations d.o.o.
(part of Sartorius),
Mirce 21, 5270 Ajdovscina, Slovenia
Aleš Štrancar
BIA Separations d.o.o.
(part of Sartorius),
Mirce 21, 5270 Ajdovscina, Slovenia
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors are employees of BIA Separations d.o.o. They have no other conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 BIA Separations d.o.o. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jan 14 2021; Revised in: Feb 19 2021; Publication date: Mar 16 2021.

