Scaling-up the production of stem cell-derived extracellular vesicles in stirred-tank bioreactors
Cell & Gene Therapy Insights 2021; 7(8), 1077–1083
10.18609/cgti.2021.140
Exosomes are extracellular vesicles (EVs), that are secreted by different cells of the body and are considered as important players in cell-to-cell communication. Exosomes, and more specifically stem cell-derived exosomes are currently of great interest as cell-free therapeutic tools due to their strong diagnostic and therapeutical potential in various diseases models. To explore their use in various biomedical frameworks, large amounts of high-quality exosomes need to be produced. To meet this challenge, cell culture in the controlled environment offered by stirred-tank bioreactor can facilitate the standardized expansion of large numbers of viable cells and enhance the reproducible production of extracellular vesicles. In this article, we discuss the challenges of exosome production and provide insights in its scale-up in stirred-tank bioreactors.
The potential of exosome technology in advanced therapies
EVs can be broadly classified, based on their mechanism of release and size, in exosomes (less than 150 nm in diameter), microvesicles/microparticles/ectosomes (100–1000 nm) and apoptotic bodies (>1000 nm) [1]Yanez-Mo M, Siljander PRM, Andreu Z et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2014; 4(1): 27066.. Originally considered as cellular waste product, exosomes are a population of naturally occurring mobile extracellular vesicles released by different cell types in vivo under various physiological and pathological conditions. They are capable of transporting various bioactive molecules such as proteins, lipids and different types of nucleic acids. Exosomes play a role in mediating intracellular communication, in addition to modulating immunoregulatory processes, tumor metabolism, regenerative and degenerative processes. Beyond their use as biomarkers, exosomes offer promising opportunities for therapeutic applications, as vehicles for drug delivery, but also as cell-free therapeutic tools [2]Hu Q, Su H, Li J et al. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020; 3(1): 54–66., [3]Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, Characterization, biological function and multifarious therapeutic approaches of exosomes. Cells 2019; 8(4): 307..
The challenges of exosome mass production
The preclinical and clinical development of exosome technology as a cell-free therapeutic and drug delivery platform requires large quantities of exosomes. The therapeutic doses required will vary depending on the specific purpose of the study and methods by which the exosomes are quantified. In earlier preclinical trials, 109 to 1011 particles were administered per animal to achieve a biological outcome [4]Haraszti RA, Miller R, Stoppato M et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018; 26(12): 2838–47.Haraszti RA, Miller R, Stoppato M et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018; 26(12): 2838–47.. Dosing is highly variable and will depend on the therapeutic target and method of delivery [5]Shekari F, Nzari A, Kashani SA, Hajizadeh-Saffar E, Lim R, Baharvand H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review. Cytotherapy 2021; 23(4): 277–84. .
Mass production of exosomes is challenging for several reasons. A delicate balance must be achieved in terms of quantity and quality. The final product must be pure and retain its biological properties. To achieve this, manufacturing processes must be scalable, controlled and standardized to improve reproducibility. Unfortunately, most of the current methods for enrichment, isolation and characterization of exosomes do not meet these criteria.
The exosome production workflow is a multi-step process composed of three main steps:
- The upstream process, in which the cells used as the exosome source are expanded and exosomes are enriched in the medium;
- The downstream process, during which the secreted exosomes are separated from the other components of the conditioned medium; and finally
- The quality control phase which is based on exosome characterization.
Considering the first step of the exosome production workflow, the upstream process leading to efficient exosome secretion, it is necessary to highlight some key factors, in order to ensure a successful and reproducible upstream process.
Extracellular vesicles are varied [6]Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Phil. Trans. R. Soc. 2017; 373: 20160479.Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Phil. Trans. R. Soc. 2017; 373: 20160479. and cell culture parameters, like the cell seeding density, passage number, cell confluence, and medium composition, can have an impact on the exosomes secreted quantity and quality [7]Patel D, Gray KM, Santharam Y, Lamichane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017; 2(2): 170–9., [8]Palviainen M, Saari H, Kärkkäinen O et al. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles 2019; 8(1): 1596669., [9]Gudbergsson JM, Johnsen KB, Skov MN, Duroux M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology 2016; 68(4): 579–92.. To achieve robust proliferation, culture media are usually supplemented with fetal bovine serum (FBS) as a source of growth factors. However, these animal-derived supplements contain large amounts of exogenous exosomes that contaminate the vesicles of interest. To avoid such contamination, xeno-free cell culture media can be used during the cell expansion and the vesicle collection steps.
The growth support on which the cells are expanded is also important. Conventional 2D culture carriers for adherent cells, such as flasks or multilayer flasks, have a limited growth area per volume. Three dimensional, microcarrier-based cell culture in a bioreactor is more physiologically representative and easier to scale up. Several teams have already published data demonstrating that 3D culture is beneficial for exosome yield and activity [4]Haraszti RA, Miller R, Stoppato M et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018; 26(12): 2838–47.Haraszti RA, Miller R, Stoppato M et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018; 26(12): 2838–47., [10]Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Nature Sci. Rep. 2019; 9(1): 13012.. The 3D culture parameters need to be optimized to ensure robust attachment to the microcarriers and subsequent cell growth. For example, different types of carriers are available, which differ among other things in the size, surface charge, and surface coating, and one needs to optimize the type of carriers to be used. The initial cell seeding volume, the ratio between the number of cells and microcarriers, and also the initial adhesion phase, for example in a reduced volume with or without agitation need to be optimized as well. Culture parameters that may affect exosome production and quality include, pH, oxygen tension, temperature, agitation, and feeding strategy. All should be kept as stable as possible and the control of dissolved oxygen and temperature should aim at mimicking the physiological conditions to avoid cellular stress.
Exosome production in stirred-tank bioreactors
Cultivation in bioreactors opens up new possibilities for process monitoring and control compared with conventional cell culture flasks. pH, temperature, and DO, among other parameters, can be monitored and controlled in real time, making it easier, on the one hand, to achieve conditions resembling the physiological situation, and on the other hand, to reproduce cultivation conditions from batch to batch.
The use of stirred-tank bioreactors offers several advantages, if an increase in the cell number is required. When using cell culture plates or flasks, increasing the number of cells usually involves ‘scale-out’, meaning increasing the number of culture vessels. Stirred-tank bioreactors also allow for ‘scale-up’, meaning increasing the culture volume by using larger vessels. In a scale-up approach less vessels need to be handled compared to the scale-out situation, which can reduce manual work and save space. Stirred-tank design allows similar vessel geometries and capabilities at different scales, facilitating a smooth transition to larger volumes. It is the bioreactor design for which most research on scale-up phenomena has been conducted and it is well established in industrial cell culture bioprocessing.
With the following example we summarize, what kind of results can be expected when using stirred-tank bioreactors. For a detailed description of the study the reader is referred to a previous publication [11]Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.. In this case, the DASbox® Mini Bioreactor System was used for the development of an exosome production process and the optimization of culture conditions at small scale, before a potential scale-up. Figure 1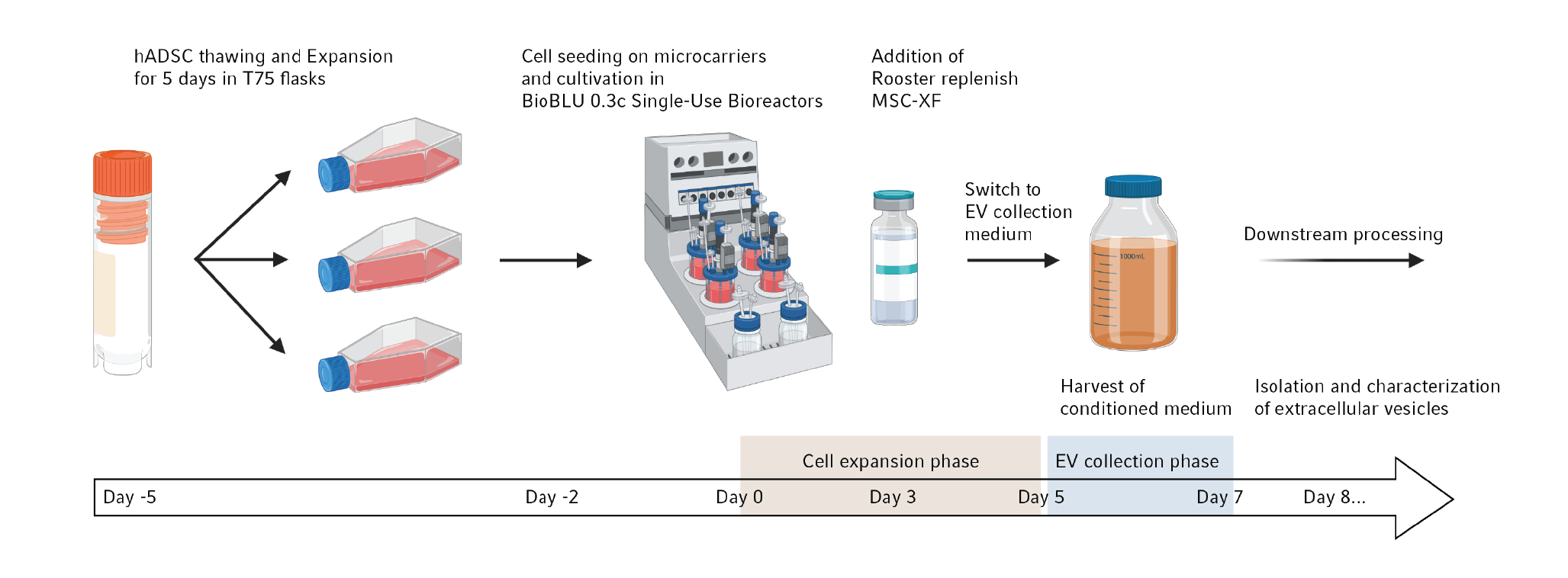
Mesenchymal stem cells derived from adipose tissue were selected as the exosome source. After thawing, the cells recovered for several days in flasks and the appropriate number was seeded on microcarriers in BioBLU® 0.3c Single-Use Bioreactors. Cell growth was monitored for 10 days. Growth factors were added 3 days after seeding. During the expansion phase, regular checks of cell adhesion and viability were performed. After 5 days of cell growth, the culture medium was completely replaced with collection medium. Exosome enrichment occurred during 2 additional days prior to harvest and subsequent downstream processing. All culture media used in this study were xeno-free [11]Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021..
The main process parameters used during this experiment have been previously summarized [11]Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.. This paper includes information on the type of microcarriers, the inoculation cell density, the final working volume, the different physicochemical parameters used, and agitation.
The analysis of cell viability using fluorescent calcein-AM and fluorescence microscopic evaluation revealed that during the five days post seeding cells grew and progressively formed small aggregates. Automated cell counting after trypsinization from the carriers confirmed the efficient proliferation with a very short lag phase. The final cell density was ca. 8.3 x 105 cells/mL, corresponding to 2.08 x 108 cells per bioreactor and a 34.7-fold increase in the cell number [11]Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021.Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021..
For downstream processing, the exosome conditioned medium was collected, centrifuged, filtered, and concentrated using Centriplus® YM 10 filter units (Amicon®). A small sample was taken for particle size distribution analysis. The remaining volume was used for size exclusion chromatography. More than 20 fractions were collected and the total amount of protein evaluated. Moreover, for each fraction we analyzed the presence of CD63 by ELISA and Western Blot analysis. As a negative control, a similar volume of non-conditioned medium has followed the same purification steps.
Figure 2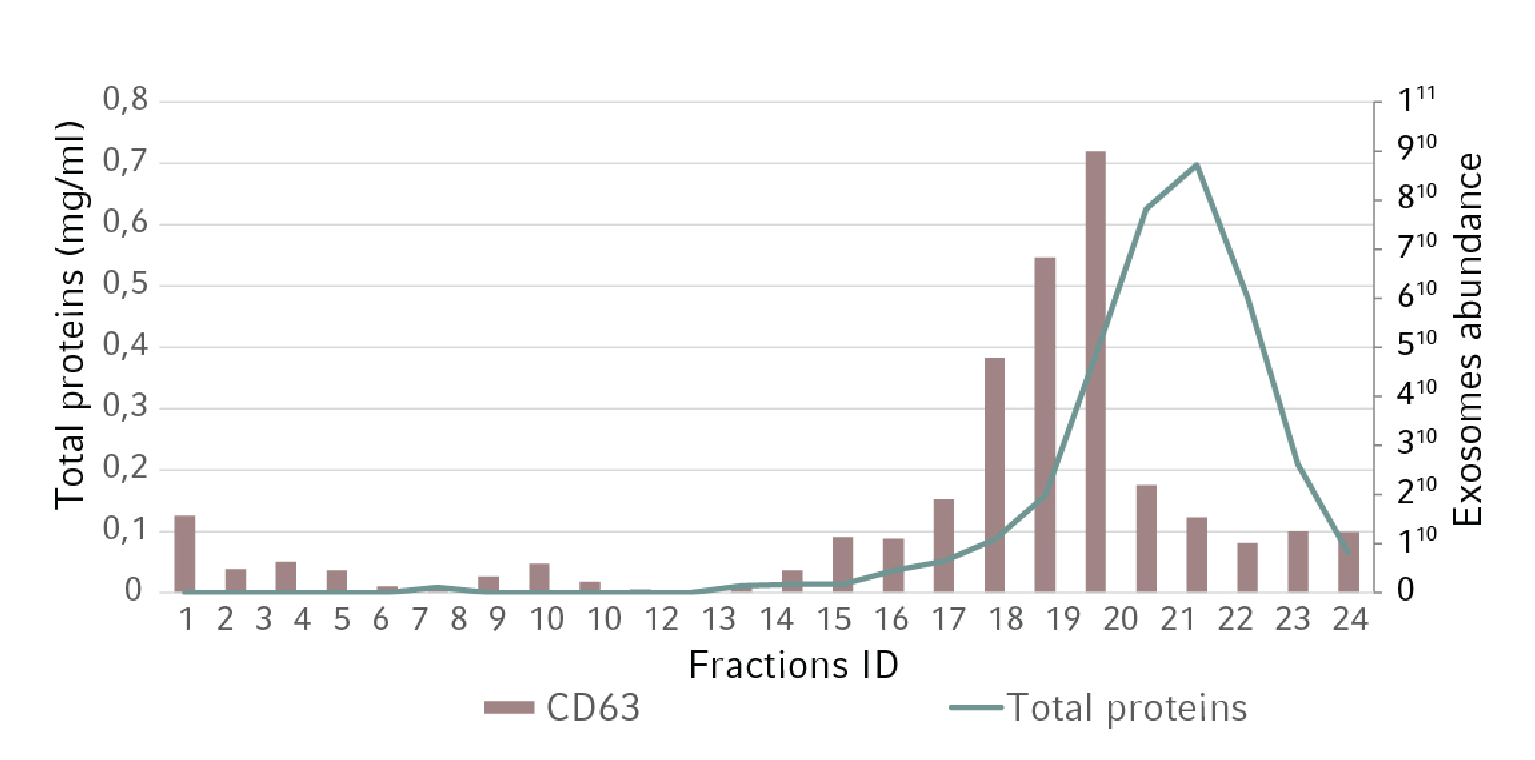
The particle size distribution was evaluated by dynamic light scattering analysis (DLA) (Figure 3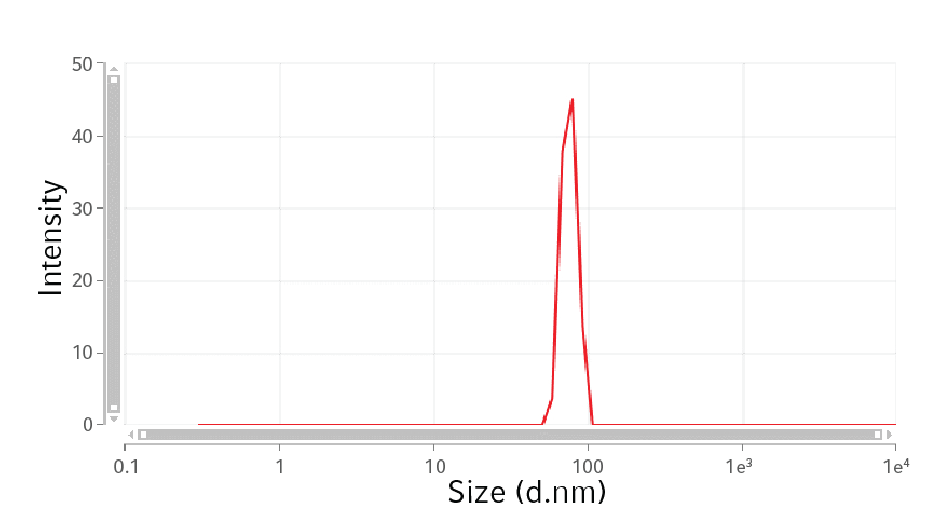
Conclusion
In comparison with conventional 2D culture platforms, bioreactors offer important advantages to optimize the upstream process of an exosome production workflow among which process monitoring, control and scalability opportunities. Following the cell expansion phase, they ensure a fine control of the main bioprocess parameters during additional incubation days in EV collection medium, ensuring optimal conditions for EV quality preservation. This study is an example highlighting the potential of hADSCs and hADSC-derived extracellular vesicle production in a stirred-tank bioprocess system.
References
1. Yanez-Mo M, Siljander PRM, Andreu Z et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2014; 4(1): 27066. Crossref
2. Hu Q, Su H, Li J et al. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020; 3(1): 54–66. Crossref
3. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, Characterization, biological function and multifarious therapeutic approaches of exosomes. Cells 2019; 8(4): 307. Crossref
4. Haraszti RA, Miller R, Stoppato M et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018; 26(12): 2838–47. Crossref
5. Shekari F, Nzari A, Kashani SA, Hajizadeh-Saffar E, Lim R, Baharvand H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review. Cytotherapy 2021; 23(4): 277–84. Crossref
6. Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Phil. Trans. R. Soc. 2017; 373: 20160479. Crossref
7. Patel D, Gray KM, Santharam Y, Lamichane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017; 2(2): 170–9. Crossref
8. Palviainen M, Saari H, Kärkkäinen O et al. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles 2019; 8(1): 1596669. Crossref
9. Gudbergsson JM, Johnsen KB, Skov MN, Duroux M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology 2016; 68(4): 579–92. Crossref
10. Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Nature Sci. Rep. 2019; 9(1): 13012. Crossref
11. Tejerina S, Dufey V, Hoet JF,Tacheny A, De Longueville F. The DASbox® Mini Bioreactor System as a tool for process development and stem cell-derived exosome production in standardized culture conditions. Eppendorf Application Note 440. 2021. Crossref
Affiliation
Aurélie Tacheny
Eppendorf Application Technologies SA, Rue du Séminaire 20A, B-5000 Namur, Belgium
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Aurélie Tacheny is employed by Eppendorf Application Technologies SA.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Eppendorf AG. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 9 2021; Revised manuscript received: Sep 15 2021; Publication date: Sep 30 2021.