Accelerating AAV capsid analysis using a new multi-capillary electrophoresis platform
Cell & Gene Therapy Insights 2022; 8(2), 231–240
10.18609/cgti.2022.039
Adeno-associated viral (AAV) vectors, while offering numerous advantages over other viruses (non-pathogenic, low immunogenicity, and can readily enter a variety of cell types), are highly complex molecules that present significant manufacturing challenges. There are a large number of serotypes to choose from, and the need to implement transfection processes that afford high yields of capsids containing the gene of interest and purification hurdles to overcome. From an analytical perspective, samples are getting more complex, more numerous, and require more complex analytical methods that involve complex method set ups, but results are needed in less time. Despite these challenges, developers of gene therapies must be able to understand the molecular liabilities of AAV vectors as soon as possible in the development process in order to ensure the manufacturability of robust, stable molecules prior to clinical trials. Existing approaches to detect and characterize product changes during drug development are part of the problem because they take too long. High-throughput analytical techniques that can overcome these complexities are becoming essential. A new system designed to enable parallel processing of eight samples simultaneously using two well-established capillary electrophoresis (CE) techniques combined with two different detection methods is filling the gap. The SCIEX BioPhase 8800 system accelerates analysis and dramatically shortens new therapy development timelines while providing the sensitive, high-resolution data expected in the biopharma industry for bioprocessing to R&D to QA/QC.
Importance of AAV purity & genome integrity
AAV is a small virus with a protein shell, or capsid, comprising three viral protein monomers (VP1, VP2, VP3) that surround a single-stranded DNA. The viral proteins have molecular weights of approximately 87, 73, and 61 kDa, respectively, totaling 60 monomers arranged in icosahedral symmetry in a ratio of 1:1:10, with an estimated size of 3.9 MDa. The DNA is approximately 4.8 kilobases in size.
To produce recombinant AAV (rAAV) vectors, host cells (typically HEK293) are transfected with three plasmids, one of which contains the entire rAAV genome and two helper plasmids that contain special Rep and Cap genes that enable the host cells to make virions. The Rep gene encodes four proteins (Rep78, Rep68, Rep52 and Rep40) with overlapping sequences that are required for gene regulation and replication of the AAV. The Cap gene encodes the three capsid proteins and a non-structural protein named AAP (assembly-activating protein). The capsid viral proteins participate in the assembly of both the capsid and genome and determine the efficacy of the gene therapy product [1]Kewal JK, Blouin V, Brument N, Agbandje-McKenna M, Snyder RO. The Role of the Adeno-Associated Virus Capsid in Gene Transfer. Drug Deliv. Syst. 2008; 437: 51–9.
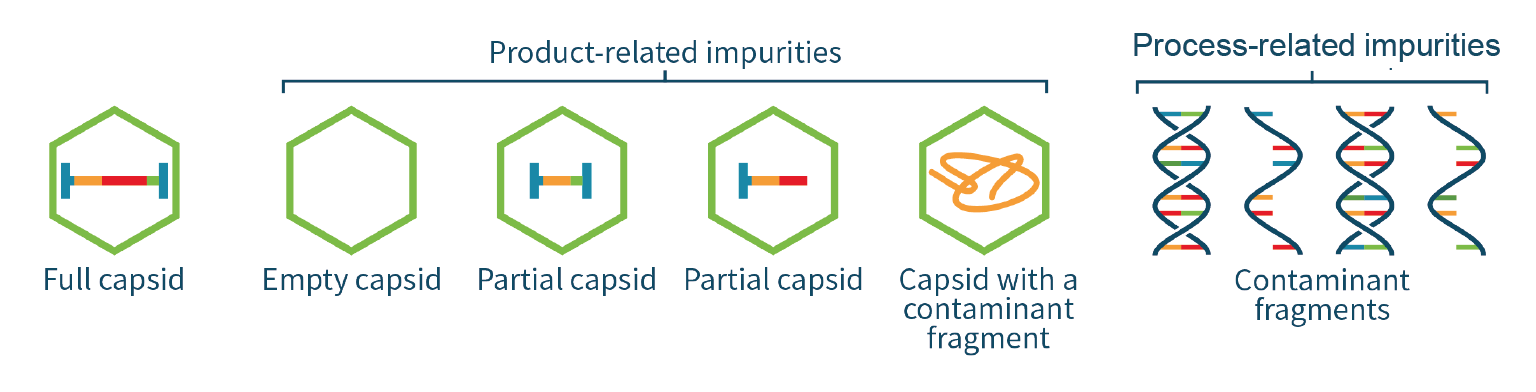
The genome of an AAV vector for gene therapy is usually composed of two inverted terminal repeats (ITR), a promoter, a trans-gene and a poly-A tail. AAV genome integrity analysis is a critical quality test for AAVs because it provides insights into transgene integrity and ensures product safety and efficacy [2]Naso MF, Tomkowicz B, Perry WL Strohl WR. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017; 31, 317–334.. It is essential that AAV capsids be expressed correctly with respect to size, peptide sequence and post-translational modifications (PTMs). Minimizing the production of capsids that do not contain the vector genome (empty) or contain truncated versions or contaminant genetic material (partial) is equally important. The purity of the capsids is also a critical quality attribute with respect to host-cell protein (HCP) and other contaminants, as they can contribute to immunogenicity and off-target effects [3]Darling S. Facilitating gene therapy development with solutions to four capsid analytical challenges. Cell Gene Ther. Ins. 2021; 7, 411–415. .
The value of CE for AAV capsid analysis
While traditional mAb-based protein therapeutics have been highly optimized for production and purification, AAVs are significantly more difficult and more expensive to produce. They typically require a multiple transfection system and produce very low titers of functional AAV. Therefore, some of the traditional techniques utilized for protein and nucleic acid analyses, such as agarose gel electrophoresis and PAGE, can be used for AAV analysis, but are quite crude compared to capillary-based methods. Capillary gel electrophoresis provides a rapid, robust and highly sensitive method for both capsid purity and genome integrity analysis, effectively separating proteins with very similar molecular weights as reflected by their migration times.
For purity analysis, CE-SDS (sodium dodecyl sulfate) offers high resolving power and excellent quantitation and reproducibility combined with automated operation and is effective even at the low concentrations of viral proteins found in AAV samples [4]Zhang C, Meagher, MM. Sample stacking provides three orders of magnitude sensitivity enhancement in SDS capillary gel electrophoresis of adeno-associated virus capsid proteins. Anal. Chem. 2017; 89, 3285–3292., [5]Sensitive AAV capsid protein impurity analysis by CE using easy to label fluorescent Chromeo dye P503. SCIEX. RUO-MKT-02-10600-A.. Detection with UV is appropriate for samples with AAV titers greater than 1 × 1013 genome copies per mL (GC/mL) or lower titers but sufficient sample volumes [4]Zhang C, Meagher, MM. Sample stacking provides three orders of magnitude sensitivity enhancement in SDS capillary gel electrophoresis of adeno-associated virus capsid proteins. Anal. Chem. 2017; 89, 3285–3292., [5]Sensitive AAV capsid protein impurity analysis by CE using easy to label fluorescent Chromeo dye P503. SCIEX. RUO-MKT-02-10600-A.. Sample labeling using fluorescent dye and laser induced fluorescence (LIF) detection can also be used to improve sensitivity of the assay.
For genome integrity analysis, CE-LIF is a rapid, automated biophysical method for genome size analysis of double-stranded DNA (dsDNA), including restriction fragment analysis of its vectors, as well as single-stranded DNA (ssDNA) and RNA and offers higher resolution than HPLC [3]Darling S. Facilitating gene therapy development with solutions to four capsid analytical challenges. Cell Gene Ther. Ins. 2021; 7, 411–415. .
BioPhase 8800 system features
The BioPhase 8800 system leverages a new cartridge that allows parallel processing of eight different CE samples simultaneously, delivering consistent, accurate results so that more samples can be analyzed in less time. Parallel processing can be achieved using either CE-SDS or capillary isoelectric focusing (cIEF) on the same or different samples containing the same or different molecules.
Accelerating AAV purity analysis
The three viral proteins in AAV capsids differ only slightly in length and the N-terminus, and each can exist as different variants with a range of PTMs, making these samples highly complex. Furthermore, the relative ratio of VP1:VP2:VP3 can be a factor impacting the potency of AAVs. The AAV protein concentrations in most gene therapies are quite low (~50 ng/mL) compared to traditional protein therapeutics. AAVs are also significantly more difficult and more expensive to produce than protein therapeutics. All of these factors taken together clearly necessitate an assay with significantly greater sensitivity and resolution than what is afforded by either agarose gel electrophoresis or SDS-PAGE. Both the PA 800 Plus and BioPhase 8800 system provide a highly reproducible and sensitive platform for AAV capsid purity analysis. The BioPhase 8800 system also provides the scalability you would expect with an 8-channel system, but also a very unique capability to dramatically accelerate method development.
To demonstrate the utility of the Bio- Phase 8800 system, a one factor at a time (OFAT) method development study was performed using AAV8 (pAV-CMV-GFP, Vigene Biosciences) samples to determine the optimal sample buffer concentration, %SDS and incubation temperature for analysis of these viral vectors. The samples were analyzed using CE-SDS-UV, and the conditions that provided the maximum peak intensity were selected as the optimum.
The application note ‘Acceleration of method optimization for AAV capsid purity analysis using multi-capillary electrophoresis platform’ [6]Li T, Mollah S. Acceleration of method optimization for AAV capsid purity analysis using multi-capillary electrophoresis platform. SCIEX Technical Note. fully describes the preparation, capsid purity analysis and data processing of the AAV8 vector. Briefly, the AAV8 capsids were chemically reduced and then diluted. The samples were separated on a BioPhase BFS Capillary Cartridge. The BioPhase software package was used for data acquisition and processing.
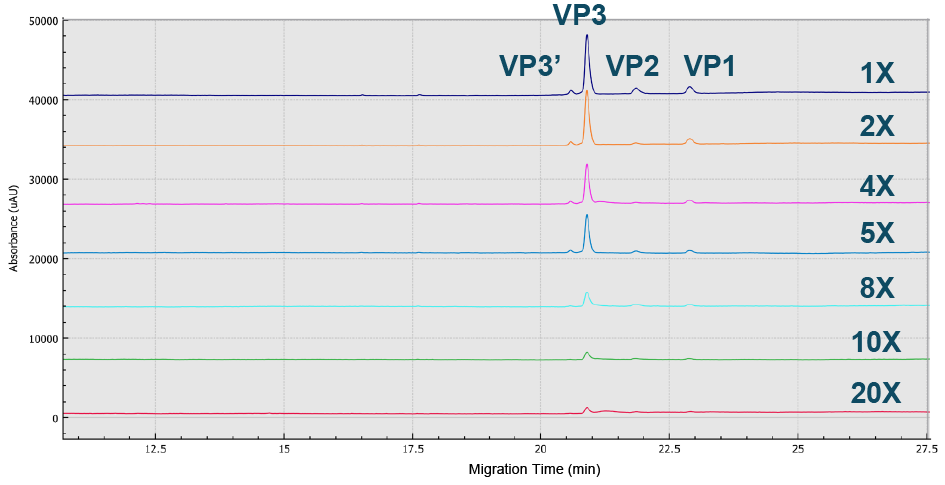
Different sample preparations and buffers were evaluated to achieve optimal sensitivity and resolution of the capsid proteins for the AAV8 serotype on the BioPhase 8800 system using CE-SDS-UV. A buffer dilution of 1× was found to be the best (Figure 1 Best peak intensity of all three AAV8 capsid proteins were obtained using a 1x dilution.Sample buffer (SDS-MW kit, 100 mM Tris-HCl pH 9.0, 1% SDS) optimization with dilutions from 1 –20 using CE-SDS-UV on the BioPhase 8800 system.), while the optimal %SDS was found to fall in the range 1–1.5% (Figure 2
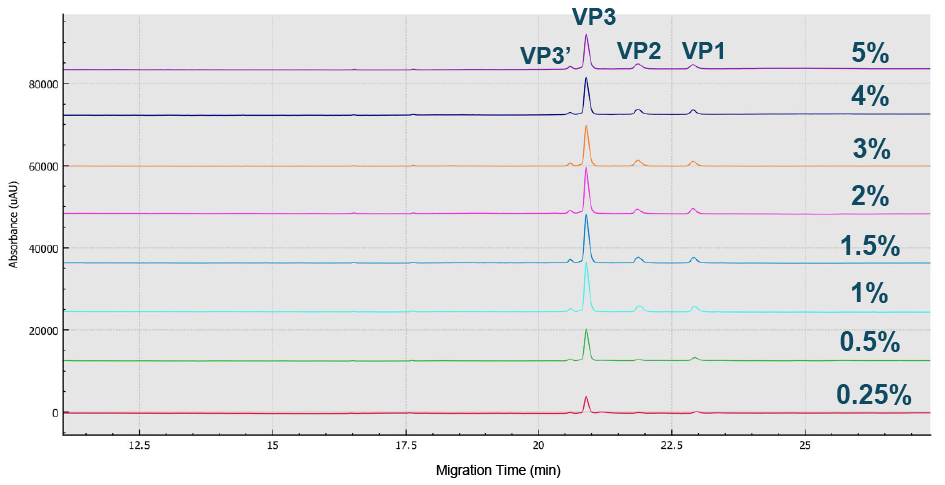
Best peak intensity of all three AAV8 capsid proteins were obtained using a 1x dilution.Sample buffer (SDS-MW kit, 100 mM Tris-HCl pH 9.0, 1% SDS) optimization with dilutions from 1 –20 using CE-SDS-UV on the BioPhase 8800 system.), while the optimal %SDS was found to fall in the range 1–1.5% (Figure 2 Optimized value obtained was 1–1.5% SDS.BioPhase 8800 electropherograms from CE-SDS-UV AAV8 sample analysis using various %SDS ranging from 0.25 to 5%.) and the peak intensity of all three capsids was optimized at 50°C (Figure 3
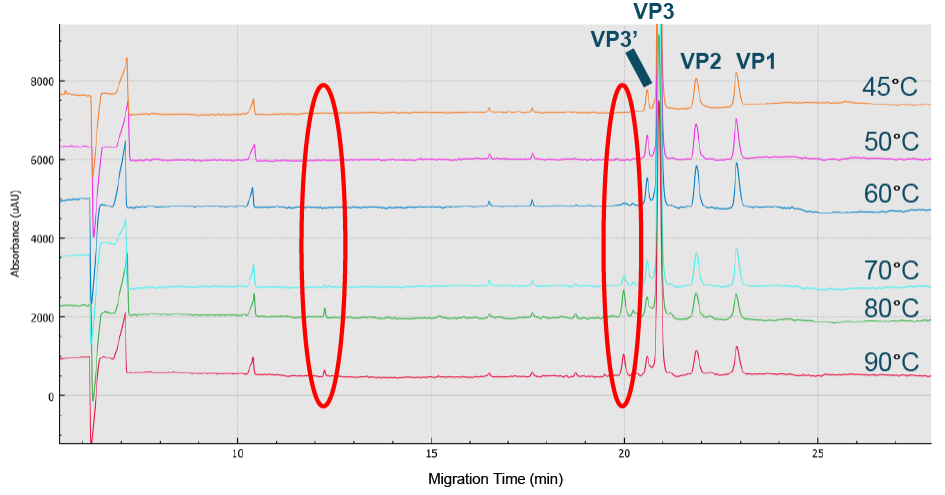
Optimized value obtained was 1–1.5% SDS.BioPhase 8800 electropherograms from CE-SDS-UV AAV8 sample analysis using various %SDS ranging from 0.25 to 5%.) and the peak intensity of all three capsids was optimized at 50°C (Figure 3 The peak intensity of all 3 capsids was optimized at 50 C.Electropherograms from CE-SDS-UV AAV8 analysis at temperatures ranging from 45 C to 90 C to determine the optimized incubation temperature.).
The peak intensity of all 3 capsids was optimized at 50 C.Electropherograms from CE-SDS-UV AAV8 analysis at temperatures ranging from 45 C to 90 C to determine the optimized incubation temperature.).
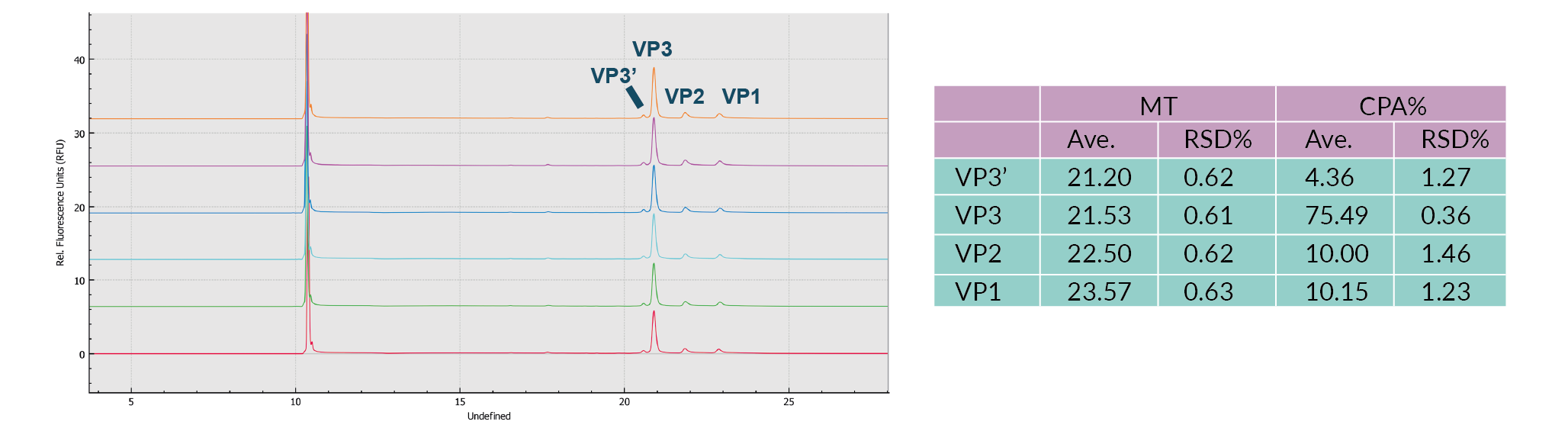
Most notably, all of the method optimization for all three of these parameters was completed in 4 hours using the BioPhase 8800 system compared to 48 hours for a single capillary system – 12 fold faster. This increase is due to each of the parallel 8 channels exhibiting highly reproducible performance across channels as well as compared to capillaries in the PA 800 Plus. To demonstrate the repeatability of analyses on the BioPhase 8800 system, results for six consecutive injections of the AAV8 sample were compared. As can be seen in Figure 4 The results were highly reproducible.Results (CE-SDS-UV) for six consecutive injections of the AAV8 sample on the BioPhase 8800 System., the relative standard deviation (RSD) of the migration time (MT) and corrected peak area (CPA)% values for the VP1, 2, 3 and VP3' (fragment of VP3) peaks were no more than 1% and no more than 1.5%, respectively.
The results were highly reproducible.Results (CE-SDS-UV) for six consecutive injections of the AAV8 sample on the BioPhase 8800 System., the relative standard deviation (RSD) of the migration time (MT) and corrected peak area (CPA)% values for the VP1, 2, 3 and VP3' (fragment of VP3) peaks were no more than 1% and no more than 1.5%, respectively.
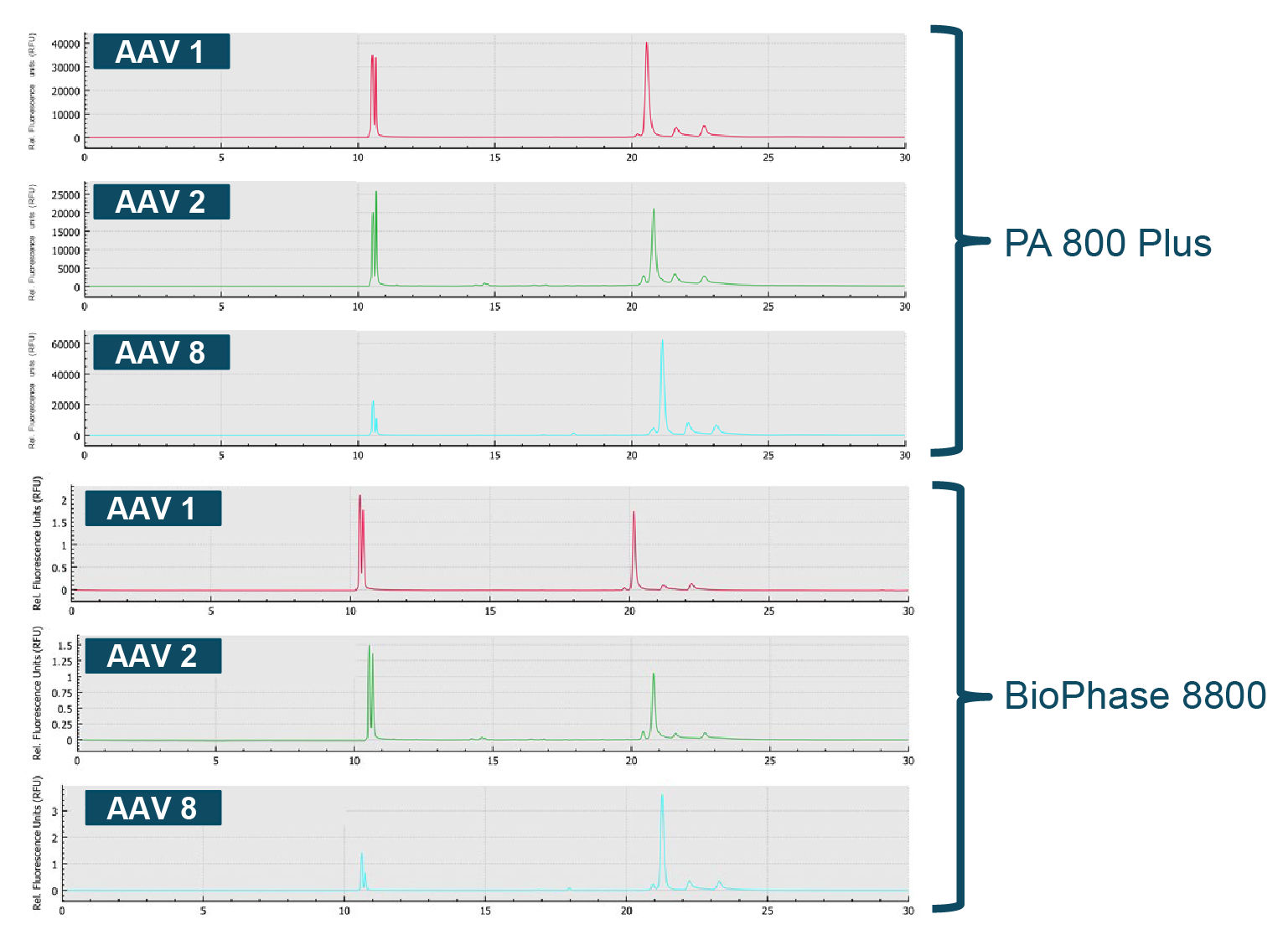
Although this example leverages a one factor at a time (OFAT) design, the BioPhase 8800 has tremendous potential to perform full design-of-experiment studies (DOE). These highly optimized assays can then be run on the BioPhase 8800 or PA 800 Plus. To illustrate this point, three different AAV serotypes (AAV1, AAV2, AAV8) were analyzed by CE-SDS-LIF on both the BioPhase 8800 and the PA 800 Plus [7]Acceleration of method optimization for AAV capsid purity analysis using multi-capillary electrophoresis platform.. The results demonstrate extraordinary correlation between the results across the two systems (Figure 5 Excellent correlation in performance across multiple AAV serotypes.Comparison of CE-SDS-LIF analysis data AAV1, AAV2 and AAV8 on the single capillary PA 800 Plus and the multi-capillary BioPhase 8800 system.).
Excellent correlation in performance across multiple AAV serotypes.Comparison of CE-SDS-LIF analysis data AAV1, AAV2 and AAV8 on the single capillary PA 800 Plus and the multi-capillary BioPhase 8800 system.).
Dramatically faster method development with the BioPhase 8800 system is also enabled by the software with advanced capabilities and drag-and-drop functionality for easy and confident method and sequence creation. The software also leverages advanced data analysis capabilities to further accelerate method development – even within a fully compliant-ready environment. Also, new validated assay kits simplify operation.
Temperature control provided on both the sample chamber and the factory-built, multi-capillary cartridge of the BioPhase 8800 system ensures maximum reproducibility by preventing degradation of the analyte(s) prior to analysis. A constant temperature in the capillaries also ensures a consistent environment for all samples and every run.
The capsid proteins of different AAV serotypes can, and frequently do, have different physical properties. Initial method development, or even worse – mid program method development, is often a significant and unpredictable delay to project timelines in an AAV therapeutic program. The unique capabilities of the BioPhase 8800 system provide a solution to avoid this project impact.
The quality of the transgene inside a viral vector impacts the infectivity, efficacy and safety of the gene therapy product. The genome cassette encapsulated in the AAV capsid could be absent, truncated, or occupied by fragments from the host-cell genome or plasmid. The analysis of AAV genome integrity is therefore of significant importance because it provides insights into transgene integrity and ensures product safety and efficacy (Figure 6 Genome impurities impact safety and efficacy.).
Genome impurities impact safety and efficacy.).
Currently, AAV genome integrity analysis by CE-LIF is performed one sample at a time using the single-capillary system. Multiplexing the analysis can help decrease the analysis or profiling time. The multi-capillary BioPhase 8800 system has been shown to effectively accelerate the execution of sensitive AAV genome integrity analysis for multiple AAV samples with different serotypes or different genome sizes while retaining the excellent resolution, sensitivity and repeatability obtained when using the single-capillary PA 800 Plus [8]Li T, Luo J, Mollah S. Genome integrity analysis of adeno-associated viruses (AAV) using multi-capillary gel electrophoresis. SCIEX Technical Note..
The application note ‘Genome Integrity analysis of adeno-associated viruses (AAV) using multi-capillary gel electrophoreses’ [8]Li T, Luo J, Mollah S. Genome integrity analysis of adeno-associated viruses (AAV) using multi-capillary gel electrophoresis. SCIEX Technical Note. fully describes the preparation, genome integrity analysis and data processing for several AAV (AAV2, AAV5, AAV9) serotypes. Briefly, AAV samples were treated to remove non-capsid genetic impurities, purified with the QIAquick PCR kit and then separated on the BioPhase 8800 system with a PVP gel-based capillary with LIF detection. Equivalent separation and analyses were performed in a single-capillary mode on the PA 800 Plus.
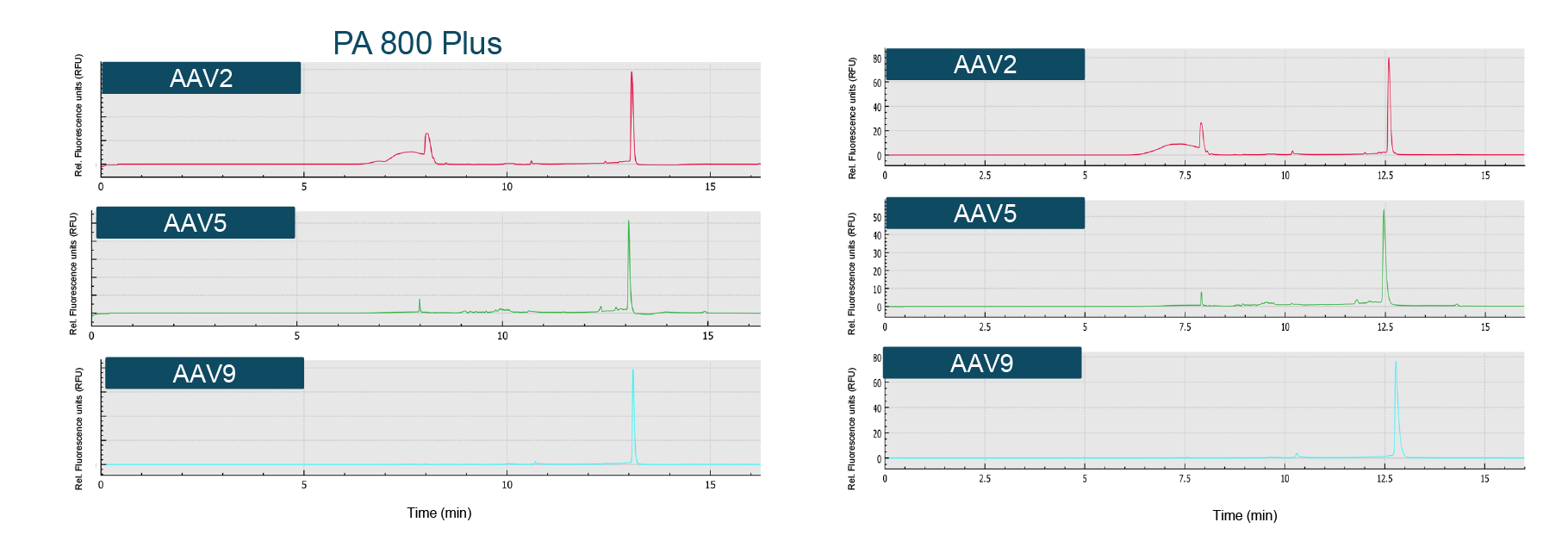
The genome profile and the migration times of the nucleic acid peaks aligned well between the two systems (Figure 7 Very good alignment of genome profile across systems.Comparison of genome integrity analysis results for AAV2, AAV5 and AAV9 on the single-capillary PA 800 Plus and the multicapillary BioPhase 8800 system using a LIF detector.). Similarly, the % corrected peak areas of the intact genome and the impurities (including truncated genome and other small sized nucleic acid impurities) correlated well.
Very good alignment of genome profile across systems.Comparison of genome integrity analysis results for AAV2, AAV5 and AAV9 on the single-capillary PA 800 Plus and the multicapillary BioPhase 8800 system using a LIF detector.). Similarly, the % corrected peak areas of the intact genome and the impurities (including truncated genome and other small sized nucleic acid impurities) correlated well.
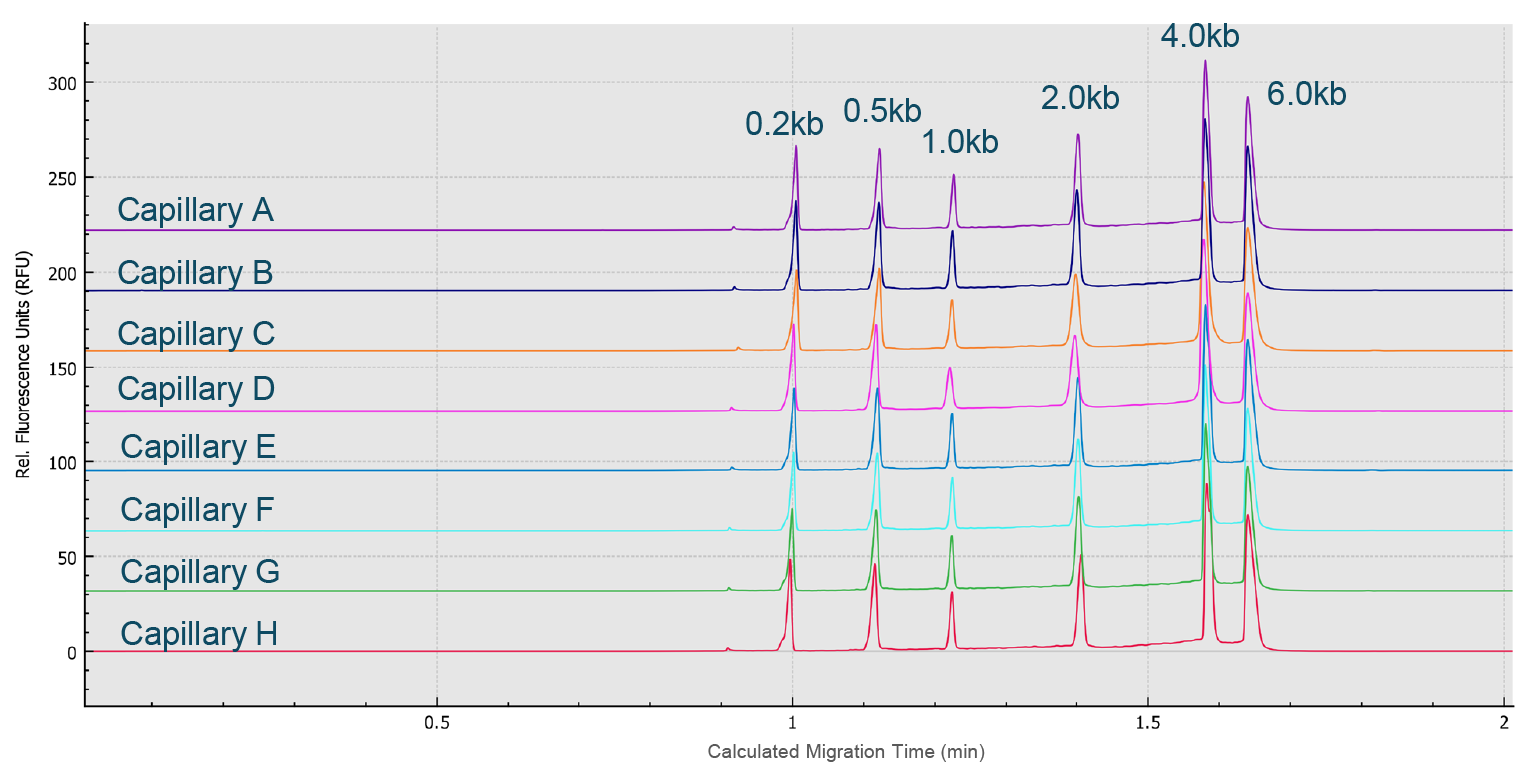
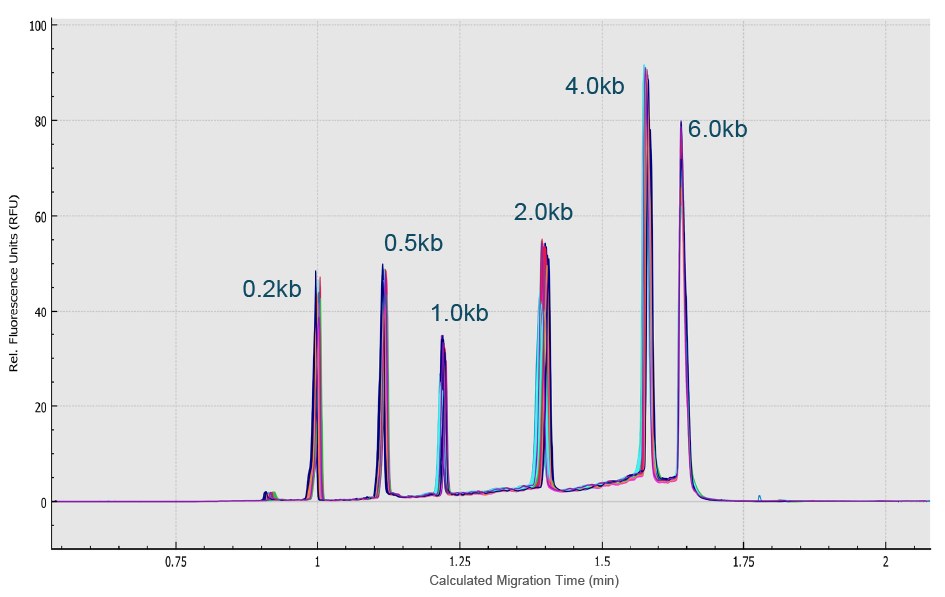
Next, an RNA ladder sample (RNA 6000 Ladder, Thermo Fisher Scientific) was used to evaluate the reproducibility of the migration time and corrected peak area values for eight analyses simultaneously performed on the eight capillaries of the multi-capillary electrophoresis system. High-resolution separation of all RNA size markers (0.2 kb, 0.5 kb, 1.0 kb, 2.0 kb, 4.0 kb, and 6.0 kb) was obtained (Figure 8 High resolution separation of all RNA size markers.Traces A to H represent the separation in the eight individual capillaries of the BioPhase 8800 system.). In addition, when ten consecutive injections of the RNA ladder sample on the eight capillaries were evaluated (Figure 9
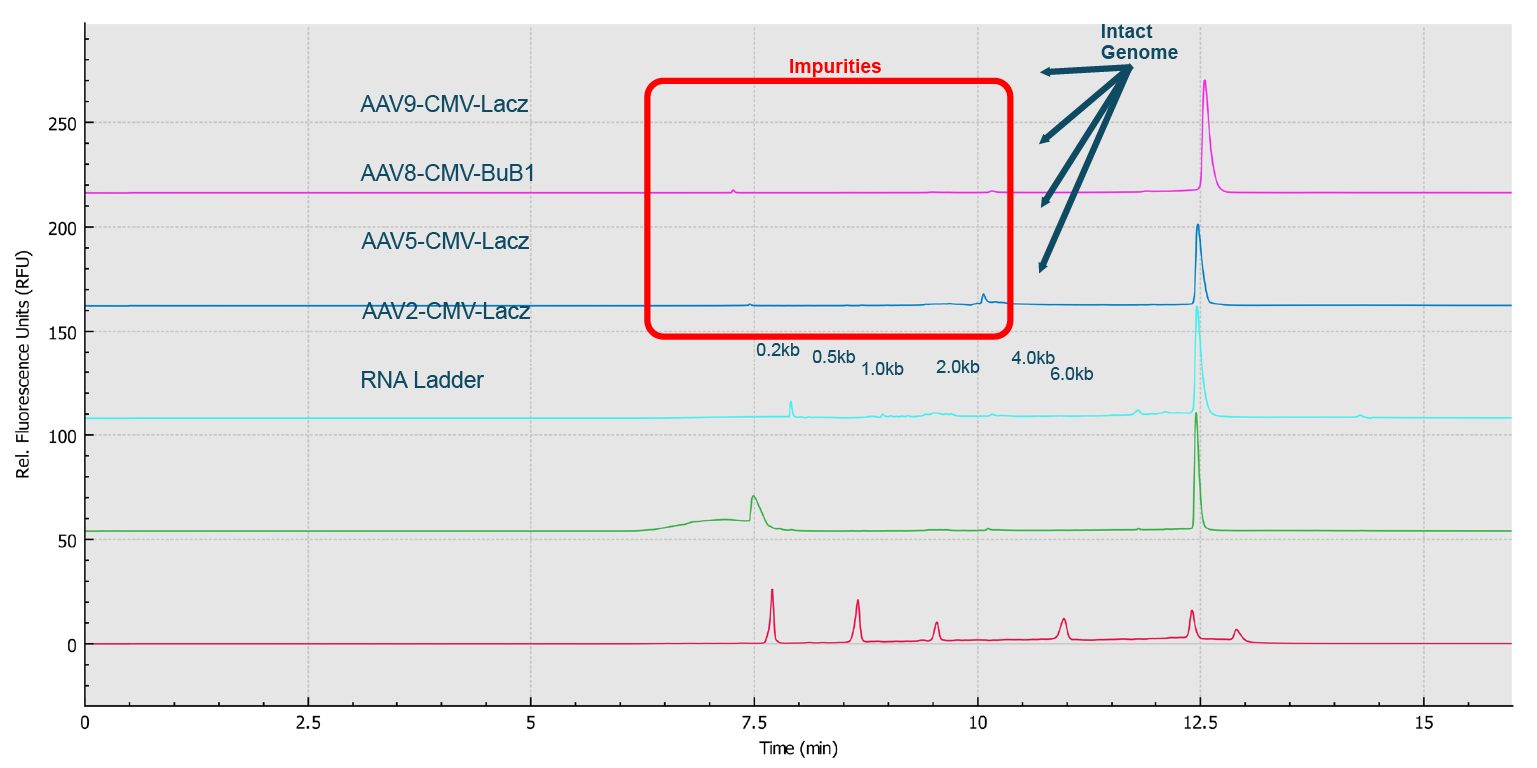
High resolution separation of all RNA size markers.Traces A to H represent the separation in the eight individual capillaries of the BioPhase 8800 system.). In addition, when ten consecutive injections of the RNA ladder sample on the eight capillaries were evaluated (Figure 9 Excellent inter- and intra-capillary repeatability.Overlaid traces of 80 injections (ten consecutive injections of eight capillary channels) of RNA size ladder on the multi-capillary electrophoresis system.), the migration time reproducibility (RSD%) of the 80 analyses for each RNA size marker was less than 1%, while the RSD% for the corrected peak area% was <5% for the RNA markers. Next, simultaneous analysis of AAV vectors with different serotypes and the same serotype with different genome sizes were simultaneously analyzed using the BioPhase 8800 system. In the first case, samples of AAV2, AAV5, AAV8 and AAV9 were analyzed in parallel along with the RNA ladder. The intact genome of AAV was well separated from the partial or truncated genome and other small size impurities for different serotypes of AAV samples (Figure 10
Excellent inter- and intra-capillary repeatability.Overlaid traces of 80 injections (ten consecutive injections of eight capillary channels) of RNA size ladder on the multi-capillary electrophoresis system.), the migration time reproducibility (RSD%) of the 80 analyses for each RNA size marker was less than 1%, while the RSD% for the corrected peak area% was <5% for the RNA markers. Next, simultaneous analysis of AAV vectors with different serotypes and the same serotype with different genome sizes were simultaneously analyzed using the BioPhase 8800 system. In the first case, samples of AAV2, AAV5, AAV8 and AAV9 were analyzed in parallel along with the RNA ladder. The intact genome of AAV was well separated from the partial or truncated genome and other small size impurities for different serotypes of AAV samples (Figure 10 Genome Integrity Analysis of AAV samples of different serotypes (Serotype 2, 5, 8 and 9) done in parallel on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Green trace: AAV2-CMV-Lacz sample. Light blue trace: AAV5- CMV- Lacz sample. Dark blue trace: AAV8-CMV-BuB1. Pink trace: AAV9- CMV-Lacz sample.). Notably, it took less than 25 min to screen eight samples using the BioPhase 8800 system. In the second case, AAV2 samples encapsulating different genome sizes were analyzed along with the RNA ladder sample (Figure 11
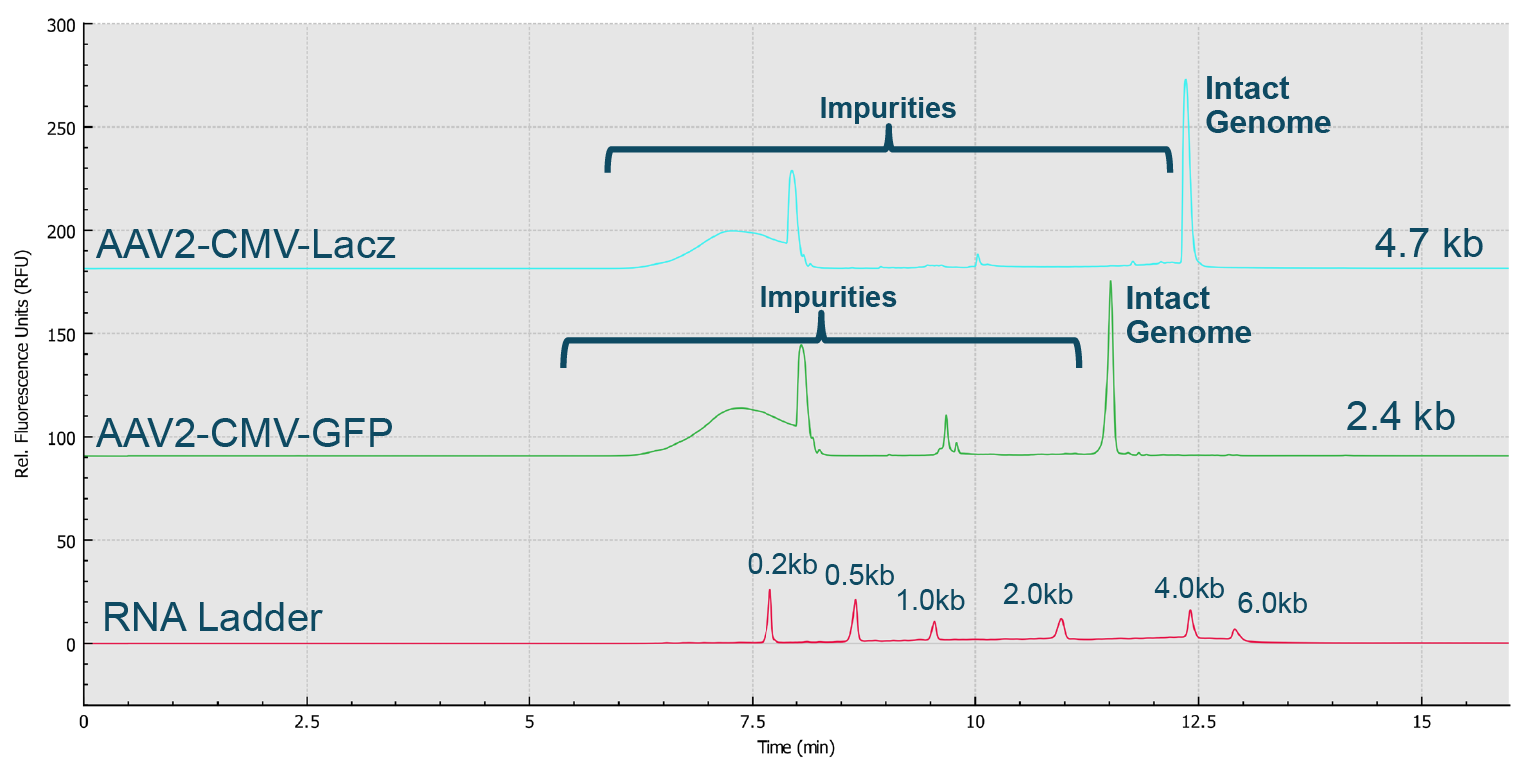
Genome Integrity Analysis of AAV samples of different serotypes (Serotype 2, 5, 8 and 9) done in parallel on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Green trace: AAV2-CMV-Lacz sample. Light blue trace: AAV5- CMV- Lacz sample. Dark blue trace: AAV8-CMV-BuB1. Pink trace: AAV9- CMV-Lacz sample.). Notably, it took less than 25 min to screen eight samples using the BioPhase 8800 system. In the second case, AAV2 samples encapsulating different genome sizes were analyzed along with the RNA ladder sample (Figure 11 Genome size analysis of AAV2 samples of different genome loads on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Green trace: AAV2-CMV-GFP sample with genome size about 2.4 kb. Blue trace: AAV2-CMV-Lacz sample with genome size about 4.7 kb.). The genome size different can be clearly seen. It is worth noting that the RNA size standards migrate slower in this PVP gel buffer than the single stranded AAV genome of the same size due to the differences in base composition in these nucleic acids, and the differences related to ribose in RNA versus deoxyribose in single stranded DNA.
Genome size analysis of AAV2 samples of different genome loads on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Green trace: AAV2-CMV-GFP sample with genome size about 2.4 kb. Blue trace: AAV2-CMV-Lacz sample with genome size about 4.7 kb.). The genome size different can be clearly seen. It is worth noting that the RNA size standards migrate slower in this PVP gel buffer than the single stranded AAV genome of the same size due to the differences in base composition in these nucleic acids, and the differences related to ribose in RNA versus deoxyribose in single stranded DNA.
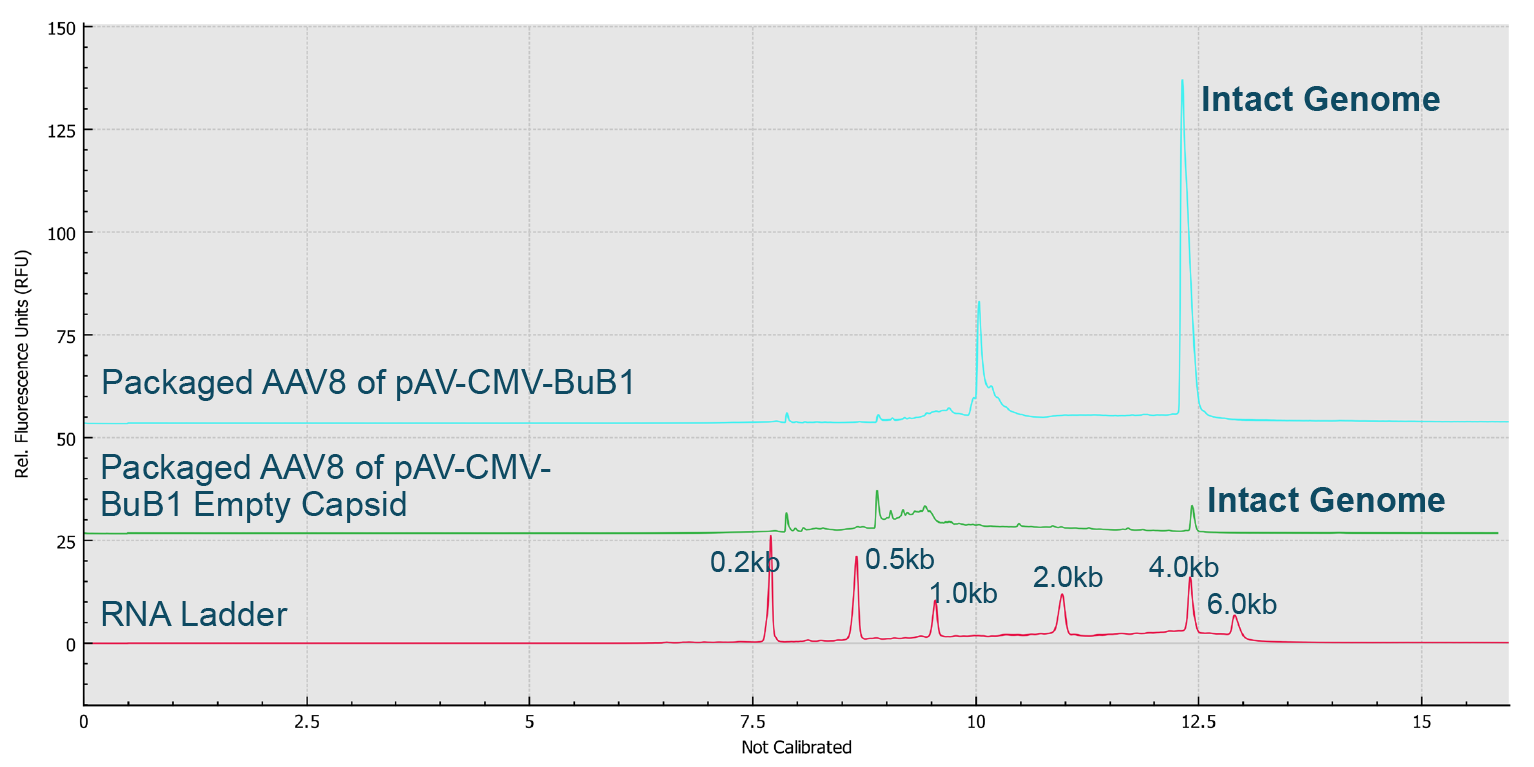
Finally, the AAV samples with enriched full and empty capsids were analyzed on the BioPhase 8800 system along with the RNA ladder (Figure 12 Genome Integrity Analysis of enriched full capsids and enriched empty capsids of AAV8-CMVBuB1 on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Blue trace: Enriched full capsids of AAV8- CMV-BuB1 sample. Green trace: Enriched empty capsids of AAV8-CMV-BuB1 sample.). The small amount of intact genome observed in the enriched empty AAV8-CMV-BuB1 sample indicated the presence of a small amount of full capsids in the enriched empty capsids sample.
Genome Integrity Analysis of enriched full capsids and enriched empty capsids of AAV8-CMVBuB1 on the BioPhase 8800 system.Red trace: RNA size standards with sizes marked in dark blue font. Blue trace: Enriched full capsids of AAV8- CMV-BuB1 sample. Green trace: Enriched empty capsids of AAV8-CMV-BuB1 sample.). The small amount of intact genome observed in the enriched empty AAV8-CMV-BuB1 sample indicated the presence of a small amount of full capsids in the enriched empty capsids sample.
Conclusion
The ability of the BioPhase 8800 multi-capillary CE system to rapidly analyze AAV capsid purity and genome integrity with the same high resolution and sensitivity well known for CE analyses on established single-capillary PA 800 Plus system was clearly demonstrated. The capability of analyzing eight AAV samples of multiple serotypes and different genome sizes at the same time on the same analytical platform can dramatically accelerate screening and process development of AAV products. The easy transferability of methods from one system to the other was also confirmed, enabling seamless movement of analyses from process development into QA/QC.
Overall, the results of these studies show that by using the SCIEX multi-capillary BioPhase 8800 system, drug developers can reduce development timelines by leveraging the efficient generation of sensitive, high-resolution data. With parallel analysis capabilities, biopharmaceutical scientists can quickly develop methods for screening and characterizing AAV vectors for gene therapies, dramatically shortening gene therapy development timelines and accelerating their time to market.
References
1. Kewal JK, Blouin V, Brument N, Agbandje-McKenna M, Snyder RO. The Role of the Adeno-Associated Virus Capsid in Gene Transfer. Drug Deliv. Syst. 2008; 437: 51–91. Crossref
2. Naso MF, Tomkowicz B, Perry WL Strohl WR. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017; 31, 317–334. Crossref
3. Darling S. Facilitating gene therapy development with solutions to four capsid analytical challenges. Cell Gene Ther. Ins. 2021; 7, 411–415. Crossref
4. Zhang C, Meagher, MM. Sample stacking provides three orders of magnitude sensitivity enhancement in SDS capillary gel electrophoresis of adeno-associated virus capsid proteins. Anal. Chem. 2017; 89, 3285–3292. Crossref
5. Sensitive AAV capsid protein impurity analysis by CE using easy to label fluorescent Chromeo dye P503. SCIEX. RUO-MKT-02-10600-A. Crossref
6. Li T, Mollah S. Acceleration of method optimization for AAV capsid purity analysis using multi-capillary electrophoresis platform. SCIEX Technical Note. Crossref Crossref
7. Acceleration of method optimization for AAV capsid purity analysis using multi-capillary electrophoresis platform. Crossref
8. Li T, Luo J, Mollah S. Genome integrity analysis of adeno-associated viruses (AAV) using multi-capillary gel electrophoresis. SCIEX Technical Note. Crossref
Affiliation
Susan Darling
Senior Director, Product Management, Market Management, CE and Biopharma, SCIEX
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author declares that they have no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 SCIEX. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Dec 7 2021; Revised manuscript received: Jan 10 2022; Publication date: Mar 16 2022.