Accelerating cell therapy discovery & development with non-viral gene engineering
Cell & Gene Therapy Insights 2022; 8(8), 1023–1032
DOI: 10.18609/cgti.2022.152
Gene engineering of immune cells is a powerful tool for creating advanced and novel cellular therapies. Currently, the critical step of cellular gene editing is primarily performed using virus-based gene delivery systems. Virus-based engineering methods are commonly plagued with long lead times, inconsistent batches, low cargo capacity, and high costs. Recently, advances in the non-viral transposon-based gene engineering have provided developers with an alternative gene engineering method that addresses virus-based platform limitations. Utilizing a ‘cut-and paste’ method of gene delivery, transposon-based systems are capable of stable genomic integration. This article discusses the transposon-based TcBuster™ platform, and its competitive advantages in cell and gene engineering.
Introduction to the TcBuster transposon system
The TcBuster transposon system for cell therapy applications incorporates gene engineering as well as expression, verification, and target validations. TcBuster is a non-viral gene editing platform aligned for stable integration of a gene of interest (GOI) into the genome. The transposition mechanism is shown in Figure 1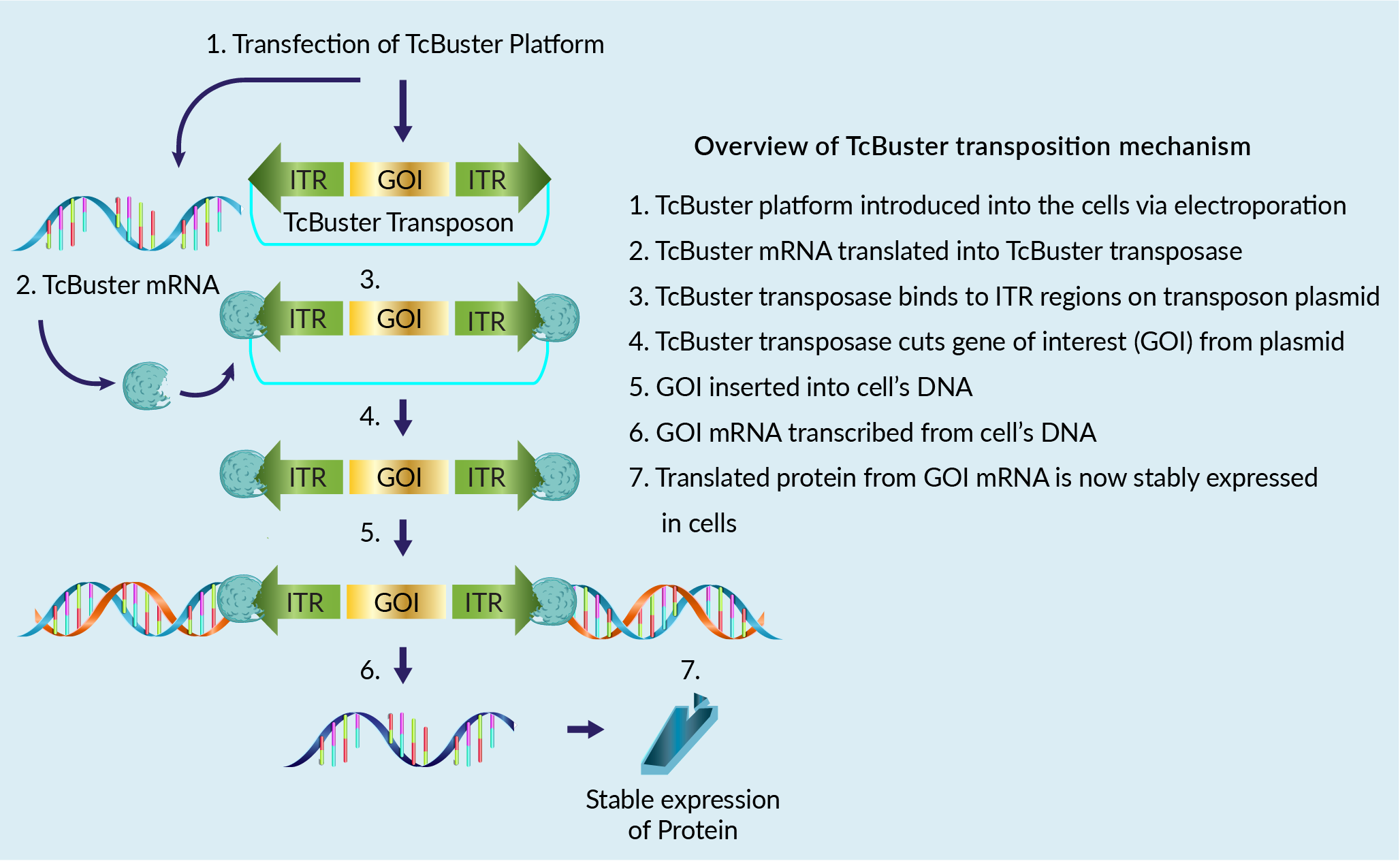 Overview of TcBuster transposition mechanism to produce stable expression of protein..
Overview of TcBuster transposition mechanism to produce stable expression of protein..
TcBuster is a part of the hAT family, one of the largest families of transposases found in nature. Successful transposon elements proliferate and diversify by vertical propagation within a single host species lineage and by horizontal transfer between species. Transposases have evolved with limited activity and mechanisms for regulation, to avoid genomic instability and excessive toxicity to their host.
TcBuster is active in mammalian cells but has low activity in T cells. Homologous transposons across different species tend to diversify, due to genetic drift and adaptation to the host species. Therefore, we have constructed a highly active TcBuster by incorporating phylogenetically conserved amino acids from related hAT family members.
Directed evolution of hyperactive TcBuster
Over one hundred consensus amino acids have been identified, and a random combinatorial library of these mutations was generated. The bulk population was tested before cells were sorted and sequenced for high performing mutants. The TcBuster transposase was manually edited to include amino acids associated with high performance to create TcBuster VE7.
To further increase TcBuster’s activity, a high-throughput combinatorial library method was used to screen for hyperactivity. These mutations were DNAse treated and randomly assembled to contain two to three alterations per enzyme. This library of TcBuster mutations was then introduced into cells along with three separate transposons, encoding green fluorescent protein (GFP), hygromycin, and mCherry. It was hypothesized that only a highly active TcBuster could stably integrate three separate transposons. Once the cells had been selected on hygromycin, the cells were sorted based on brightness for GFP and mCherry. The bright-cells were isolated and sequenced to identify individual active TcBuster mutations.
Promising TcBuster mutants were screened in HeLa cells. Most mutants were shown to perform better than wild-type TcBuster. TcB-M was shown to be the most active TcBuster mutant identified in the screen, increasing transposition efficiency from 20% to over 60%.
The stringency of the screen was increased in T cells, using a tri-cistronic transposon induced experiment. It was further hypothesized that only a highly active TcBuster could stably integrate large cargo in primary T cells. The mutants were tested in T cells in comparison to VE7. Most mutants performed similarly to VE7, however, TcB-M clearly outperformed others.
TcB-M activity in primary T cells was further analyzed using three separate multi-cistronic vectors. In every instance, significantly higher transposition efficiencies were observed with TcB-M compared to VE7. This data was reproduced multiple times in multiple donors, and as a result, TcB-M is now the leading hyperactive TcBuster for cell and gene therapy. It is currently in use by our Custom Gene Engineering Services and is expected to enter clinical application in the near future.
TcBuster in cell therapies
Increasingly complex cell therapies require a larger cargo capacity, which renders lentivirus unsuitable on its own. Many people are looking for transposon systems to help integrate larger copies.
The editing workflow for both T cells and natural killer (NK) cells includes activation, transposition, and expansion. In a comparison of transposition versus transduction using lentivirus in T cells, it was shown that TcBuster editing efficacy is as high as that of lentivirus (Figure 2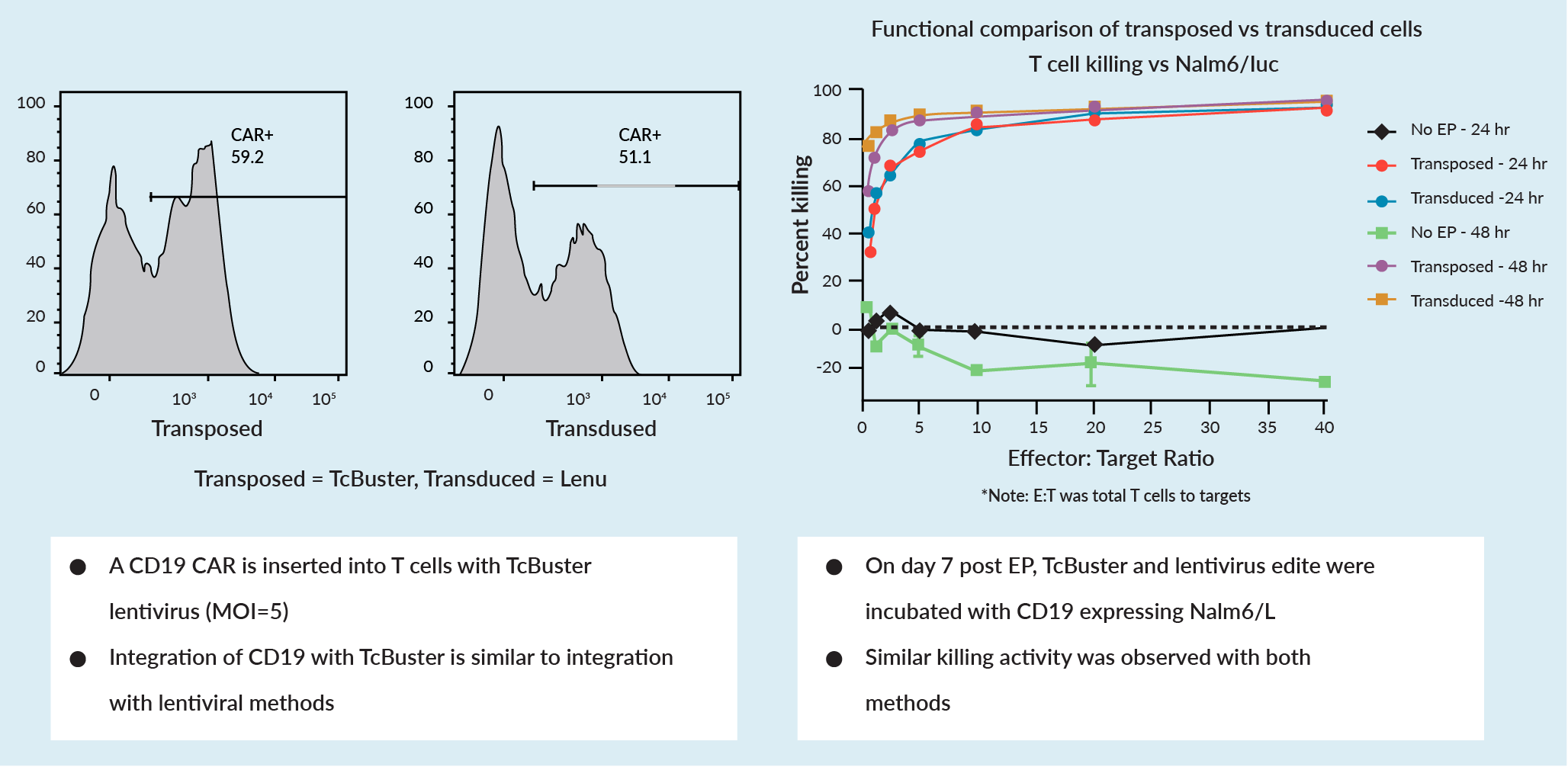 Comparison of transposed (TcBuster) versus transduced (lentivirus) cells.).
Comparison of transposed (TcBuster) versus transduced (lentivirus) cells.).
Scalability
The TcBuster platform can be optimized to support different research and therapeutic scales. TcBuster can be used at small-scale, mid-scale, and large-scale reactions without any drop off in terms of efficiency or cell expansion.
In the small-scale workflow, 10 million cells are electroporated to yield 250–350 million cells in a G-Rex™ 6M plate. At the mid-scale, 80 million cells are electroporated to yield two–three billion cells as an output. The large-scale electroporation of 400 million total cells, yields approximately 15 billion total cells. Folds roughly the same for each of these scales.
Experiments were used to compare their capabilities for transgene expression. A major consideration is to ensure that transposition efficiency is stable at scale. Equal transposition efficiency is found across all scales, suggesting that any difference between proteins in terms of expression is likely due to the detection reagents used rather than scale.
Single electroporation for transposition
A single electroporation experiment was conducted for both transposition and knockout (Figure 3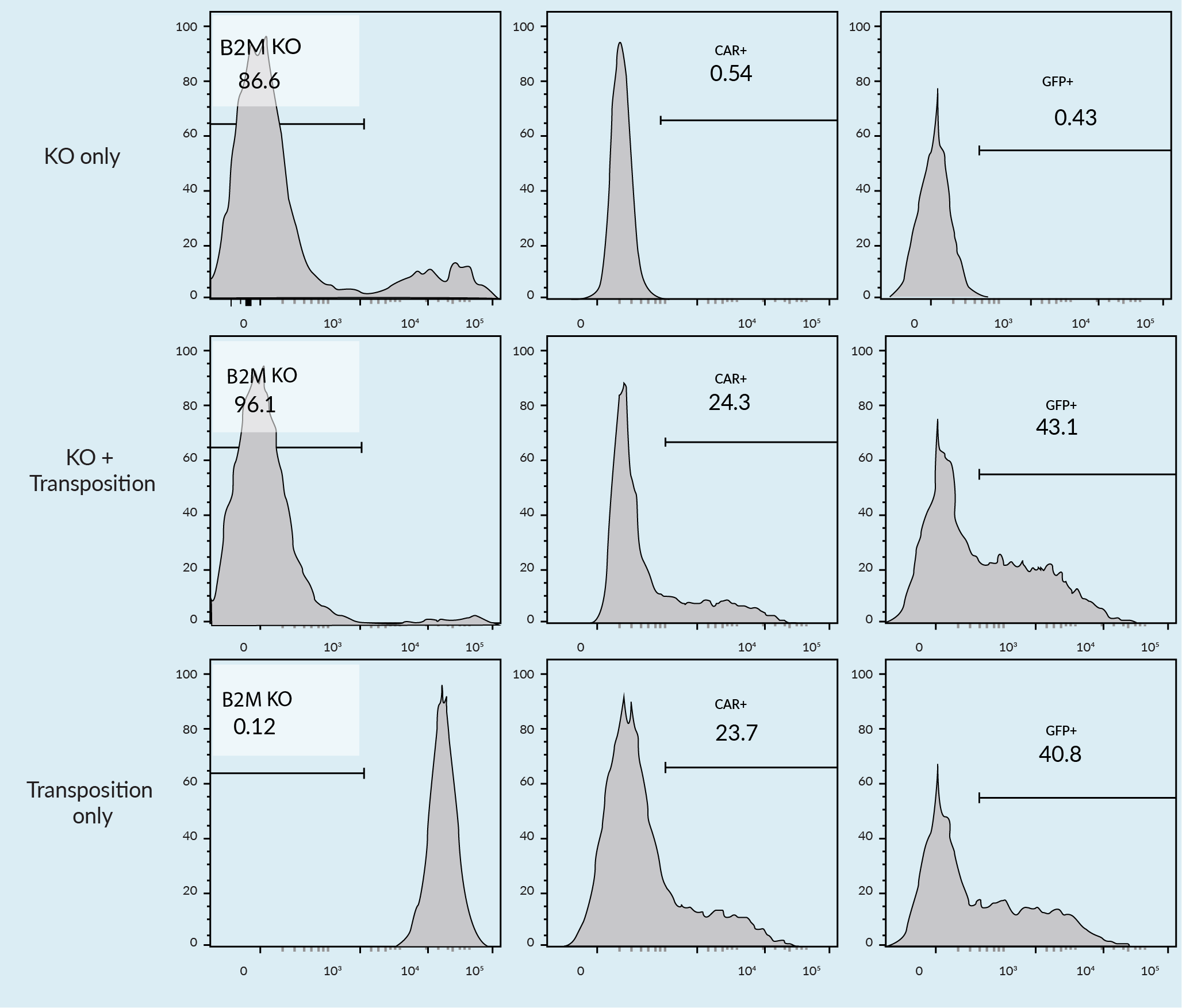 Combination of transposition with beta 2M knockout NK cell transposition.). Beta 2-Microglobulin knockout was successfully completed with a transposition of a CD19-GFP bicistronic chimeric antigen receptor (CAR). With the TcBuster system, a one-step process can be used to perform knockouts and TcBuster-mediated gene delivery without sacrificing growth and integration efficiency. These results indicate that the addition of clustered regularly interspaced short palindromic repeat (CRISPR) reagents, guide RNA, transposon, and TcBuster reagents, can be performed in a single electroporation, efficiently conducting a knockout and a knock-in, in a single unit operation.
Combination of transposition with beta 2M knockout NK cell transposition.). Beta 2-Microglobulin knockout was successfully completed with a transposition of a CD19-GFP bicistronic chimeric antigen receptor (CAR). With the TcBuster system, a one-step process can be used to perform knockouts and TcBuster-mediated gene delivery without sacrificing growth and integration efficiency. These results indicate that the addition of clustered regularly interspaced short palindromic repeat (CRISPR) reagents, guide RNA, transposon, and TcBuster reagents, can be performed in a single electroporation, efficiently conducting a knockout and a knock-in, in a single unit operation.
NK cell transposition
CD19 CAR transposition in NK cells has been performed on many donors, and results typically range between 20–40%, respective of the donor. Expansion of transposed NK cells ranges from ~250–1,000-fold in 20 days, dependent upon donor and feeder line. Overall, good expansion has been shown with cell viabilities typically greater than 95%.
Transposed NK cells also demonstrated good cytotoxic potential against both K562 cells and CAR-specific killing of CD19-positive targets (NALM6 cells), as shown in Figure 4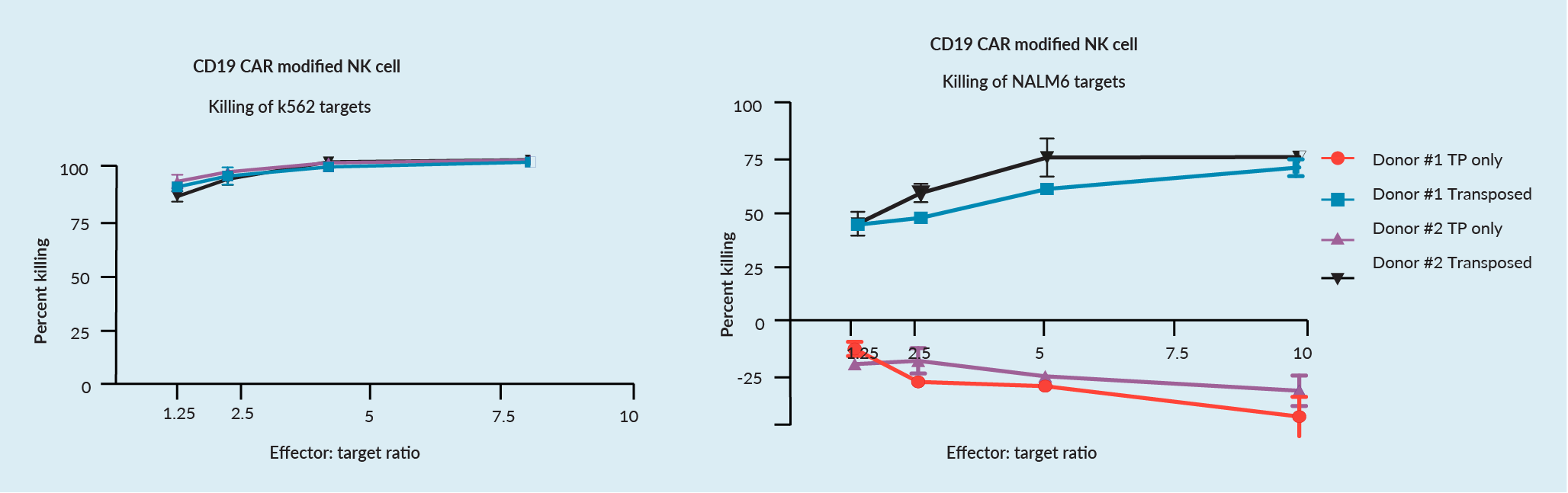 The augmented capability of NK cells to kill CD19-positive NALM6 luciferase modified targets and K562 targets.. It is important that TcBuster does not inhibit the natural killing ability of NK cells, as this adds value in its use within cell therapies.
The augmented capability of NK cells to kill CD19-positive NALM6 luciferase modified targets and K562 targets.. It is important that TcBuster does not inhibit the natural killing ability of NK cells, as this adds value in its use within cell therapies.
Advantages & applications of TcBuster
Target site integration analysis has revealed that the TcBuster transposon system has a safer insertional profile than lentivirus. The median distance to the transcriptional start site was found to be greater than that of lentivirus, suggesting less potential for influencing endogenous genes. Insertional location is less likely to be within intron regions compared to lentivirus. With lentivirus, more exon and intron insertions were seen, with TcBuster locating closer to that of the random theoretical control.
Further experiments were performed to assess the potential for clonality. From an Food and Drug Administration (FDA) regulatory perspective, it is undesirable for a single integration clone to grow out, because it may indicate a transformed T cell. Low frequency of a single integration site indicates that the final cell population has a diverse set of integration events indicating a diverse initially edited population. TcBuster clones showed a diverse integration profile, suggesting a lack of clonal outgrowth.
TcBuster is moving closer to use in the clinical setting. Luminary Therapeutics has filed for an Investigational New Drug application (IND) using TcBuster in the clinic for a B-cell activating factor (BAFF) ligand-based CAR T cell therapy, that targets three receptors expressed in B cell cancers. TcBuster has also been shown an efficient method to engineer and produce primary CAR NK cells targeting CLL-1 for acute myeloid leukemia (AML).
Further, TcBuster can be utilized in induced pluripotent stem cell (iPSC) applications. The platform was implemented in a gene replacement project to engineer iPSCs with a gene under a constitutive promoter. The median distance to the transcriptional start site and insertion location are similar in iPSCs and primary T cells. The desired clones can be selected via genetic screening.
Other applications of TcBuster include increasing antibody production titer, while simultaneously reducing manufacturing timeline. Using TcBuster internally at Bio-Techne, a 32-fold increase in CD3 production and a 58-fold increase in CD28 expression was achieved. TcBuster has also been utilized to generate a K562-based stable feeder cell line for primary NK cell culture needs. It can also generate stable cell lines for drug discovery workflows, either as population or high-expression clones.
Future directions
Bio-Techne intends to provide TcBuster as a protein. Additionally, we strive to continually enhance the TcBuster activity profile, to more efficiently deliver larger packagers. Another goal is to leverage the properties of TcBuster as a fusion protein. GFP can be fused to the TcBuster without any loss in transposition efficiency, which enables the potential of improving its integration profile and specificity.
Q&A
David Hermanson (left) and Scott Silaika (right), Director, Commercial Business Development, Cell and Gene Therapy, Bio-Techne, answer questions about the TcBuster platform.
Is there a GOI size limit for TcBuster, and is the integration site-specific?
DH: The size limit for TcBuster is based on the transfection technologies. We found that electroporation generally cannot get a plasmid larger than 10–12kb into the cell. TcBuster itself can transfer large cargoes over 10–12kb. However, practically speaking, when transfecting T cells or NK cells, getting plasmids much larger than 10–12kb starts to become a challenge.
The integration is not site-specific. It is a random integration, though we do have thoughts on how to skew that integration profile and could eventually perhaps make it site-specific.
How does TcBuster compare to other non-viral systems such as Sleeping Beauty or PiggyBac?
DH: We have done some limited direct comparisons between TcBuster and Sleeping Beauty and have found that the TcBuster does have higher integration rates than the Sleeping Beauty SB100. We have never done a direct comparison to piggyBac to date, especially with some of the hyperactive mutants that are being utilized by Poseida Therapeutics.
We could compare it to the literature data of integration profiles. Out of the three transposons, Sleeping Beauty is more random than TcBuster. PiggyBac is slightly more preferential to active chromatin. All of them show more random integration than a lentivirus.
How many copies are integrated into the genome and what is the variability in copy number from cell to cell?
DH: We have done copy number analysis using droplet digital PCR. For our populations, our copy number is found to be around three–five in T cells, and slightly lower in NK cells at two–three copies per cell range in terms of the entire population. With T cells and NK cells, we have not analyzed individual cells to evaluate copy numbers, so we do not currently have the data on variability.
How can TcBuster be evaluated by interested parties?
SS: There are two methods to start evaluation. One would be a proof-of-concept study at Bio-Techne, where we gauge with potential clients and test their cells using TcBuster, providing the final frozen cells, the protocol, and the test results.
The other is a material transfer agreement, where we provide the transposase and either standard transposons such as CD19 or GFP, or custom transposons with the GOI. This can be done quickly and relatively inexpensively for proof-of-concept.
Is there a license fee for the use of TcBuster?
SS: There is a fee. The business model has a few different components, and is milestone-based, as the clients progress through the clinic. There is a royalty associated with the use of a commercial basis. We try to keep it reasonable up front, until both our customers and eventually, ourselves will be successful commercially. We are happy to discuss this further.
Has TcBuster been used in the clinic?
SS: Our first clinical use will be soon, within a matter of months. The IND is already approved.
Have you tried any transfection technologies other than electroporation?
DH: We are always interested in evaluating the squeeze technologies. We have investigated some of the other technologies such as the SE cell Line or Tito Pen, as well as lipid nanoparticles.
We do not have any concrete data that I am able to share, but it certainly is something that we have hopes for. One of the major limitations in terms of getting the cargo in and the size of the cargo comes down to the transfection technology. If we can move away from electroporation into another transfection modality that allows for larger plasmids, then our ability to transfer even larger cargoes will improve.
Does Bio-Techne offer off-the-shelf TcBuster GFP to test or use as a control for initial feasibility studies?
SS: We have standard CD19 GFP constructs that we can provide quickly.
How do you assess days post-NK activation for best transposition?
DH: Normally, our transposition rates in NK cells are most dependent on the method of growth. We tend to try to think up our transgene expression with the activation. For example, if we are using K562s as a feeder line, stimulating them every 7 days, typically we will look at the transgene expression one–two days after stimulation. You will see some variability seven days after stimulation, and the transgene expression percentage will be lower than two–three days after stimulation. That holds true through multiple rounds of stimulation. There is part of the cell cycle that comes into play in terms of detecting it.
How would you say this approach compares with the latest advances in the lentiviral space for CAR T cell generation?
DH: In terms of the phenotype for transposed cells versus transduced cells, in general, transposon systems lead to a higher percentage of memory cells. This is related to how the mechanism of transposons perform better in naïve cells than in the effector populations from the apheresis product.
Have you compared with CRISPR/Cas9, and if so, are there any advantages with TcBuster?
DH: We have not a done head-to-head comparison, though we have worked with customers who are evaluating them side by side. The integration rates tend to be higher using TcBuster than with a CRISPR/Cas9 directed site integration. The CRISPR/Cas9 mechanisms work acceptably for relatively small cargo. However, as you move to larger cargo, we have seen the rates fall off significantly.
Does copy number increase with cell propagation, or is it limited by turnover of transposase?
DH: Transposition occurs in the first 48–72 hours after electroporation. After that point, the transposase mRNA as starts to become undetectable by a qRT-PCR reaction. After the first 72 hours, there is no more transposition occurring, so the copy number does not continue to increase.
The integrations are also stable long-term. We have taken T cells and continued to activate them through CD3/CD28 bead activation to the point where the T cells no longer expand ex vivo; we still maintain the same level of integration throughout. There is no difference in copy number whether you run it 7 days post-transposition or after three–four rounds of stimulation.
Can you clarify why GFP affects Sleeping Beauty but not TcBuster mechanisms?
DH: Some people have tried to fuse GFP onto both the N terminal and C terminal of Sleeping Beauty, and it has been shown to prevent all transposition activity. We have been able to fuse GFP to TcBuster and still get equal transposition efficiency. It seems to be an inherent property of the enzymes.
What is the cost of transposition versus LV transduction per sample?
DH: In a clinical-scale manufacturing run, the cost of lentivirus can be anywhere from about $15,000–$25,000 depending on lentivirus run efficiency. We are targeting <$10,000 per patient cost of goods for both the transposon and transposase.
Affiliations
Xiaobai Patrinostro PhD
Manager of Genome Engineering Services Cell and Gene Therapy,
Bio-Techne
David Hermanson PhD
Senior Manager of R&D Applications in Cell and Gene Therapy,
Bio-Techne
Scott Silaika
Director,
Commercial Business Development, Cell and Gene Therapy,
Bio-Techne
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Dr Hermanson is a Bio-Techne stockholder.
Funding declaration: Dr Patrinostro received SBIR grant solicitation # PA17-302; awarded 2019-06-01 to 2021-05-31.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Bio-Techne. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a webinar, which can be found here.
Webinar recorded: Jun 16 2022; Publication date: Sep 26 2022.