IsoTag™AAV: an innovative, scalable & non-chromatographic method for streamlined AAV manufacturing
Cell & Gene Therapy Insights 2022; 8(10), 1287–1300
DOI: 10.18609/cgti.2022.190
Demand for gene therapies capable of treating previously inaccessible targets has risen precipitously in the past decade. Adeno-associated viruses (AAVs) are the preferred vector for gene delivery because of their favorable safety profile and tissue tropism, but they have significant manufacturing challenges, with end-to-end yields as low as 10–30%. To combat these low yields, we developed IsoTag™AAV, a novel purification technology for AAV that is a departure from the chromatographic paradigm in downstream processing. This proprietary technology uses a self-scaffolding recombinant protein reagent that can improve manufacturing yields. It enables purification by cost-effective and scalable filtration processes and improves product quality with minimal optimization. Herein, we describe the development of IsoTag™AAV, provide a head-to-head comparison to industry-leading affinity chromatography (evaluation carried out through a joint research project with Capsida Biotherapeutics), and demonstrate how it can reduce cost of goods for a clinical AAV program by 25%.
Introduction
The recent acceleration of the gene therapy industry has created hope of curing patients of once seemingly incurable diseases. However, these therapies rely on recombinant versions of naturally occurring viruses that have significant drawbacks in terms of their safety profile and manufacturability compared to the previous generation of protein-based biologics [1]Gupta V, Lourenco SP, Hidalgo IJ. Development of Gene Therapy Vectors: Remaining Challenges. J. Pharm. Sci. 2021; 110(5), 1915–1920. [2]Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr. Gene Ther. 2013; 13(6), 453–468. [3]Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J. Transl. Med. 2016; 14(1), 288. [4]Zhou HS, Liu DP, Liang CC. Challenges and strategies: the immune responses in gene therapy. Med. Res. Rev. 2004; 24(6), 748–761.. Adeno-associated virus (AAV) is the most widely used vector for gene therapy because of its superior safety profile. However, low AAV expression titers, highly inefficient purification, and an evolving regulatory landscape are slowing development and casting doubt on the commercial feasibility of gene therapies. Regulatory and social developments are also increasing the pressure on gene therapy companies to deliver affordable products with additional attention to quality, safety, and efficacy. The industry is in desperate need of more efficient and scalable methods to accelerate development, alleviate manufacturing bottlenecks, and improve global access to these life-saving therapies.
The industry has taken several approaches to address the AAV bottleneck. By engineering cells, the industry hopes to improve viral titers, thereby reducing the reactor volume required for each dose [5]Aucoin MG, Perrier M, Kamen AA. Improving AAV vector yield in insect cells by modulating the temperature after infection. Biotechnol. Bioeng. 2007,97(6), 1501–1509. [6]Merten OW. AAV vector production: state of the art developments and remaining challenges. Cell Gene Ther. Insights 2016; 2(5), 521–551. [7]Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998; 72(3), 2224–2232.. Other researchers are seeking to improve the potency by engineering the capsid to have more desirable tissue tropism or engineering the promoter for higher or tissue-specific payload expression. Both solutions are attempts to reduce the required dose and thereby the respective production volume per dose. Finally, there has been a brute force effort worldwide to improve capacity by scaling out manufacturing capability.
New or expanded manufacturing facility announcements have frequented the headlines in the past several years. Billions of dollars have been invested in increased viral vector manufacturing capacity. Despite these efforts, the downstream bioprocessing segment has seen little improvement and a dearth of innovation. The lack of standardization and low yielding unit operations result in major inefficiencies and time-consuming development, leading to costly clinical campaigns [8]Singh N, Heldt CL. Challenges in downstream purification of gene therapy viral vectors. Curr. Opinion Chem. Eng. 2022;35. [9]Fuerstenau-Sharp M, Pettitt D, Bure K, Smith J, Ware J, Brindley D. Scalable purification of viral vectors for gene therapy: An appraisal of downstream processing approaches. BioProcess Int. 2017; 15(2). [10]Capra E, Gennari A, Loche A, Temps C. Viral-vector therapies at scale: Today’s challenges and future opportunities. McKinsey Insights 2022.. Affinity chromatography – currently the dominant methodology to purify AAV – was invented for small molecules that are 10,000X smaller and less complex than AAV [11]. It is not well suited for large, complex, and fragile viruses under development today. This has contributed to the inefficiencies, supply-demand imbalance, and high cost of goods in the cell and gene therapy industry.
IsoTag™AAV is a technology that was developed with the unique needs of viral vectors in mind. The technology is a major departure from the current bioprocessing paradigm, leveraging the phenomenon of liquid-liquid phase separation that was evolved over billions of years ago by nature to perform complex cellular separations [12]Kraemer EO, Lansing W. The Molecular Weight of Linear Macromolecules by Ultracentrifugal Analysis. I. Polymeric i-Hydroxydecanoic Acid1. J. Am. Chem Soc. 1933; 55(10), 4319–4326. [13]Stein R, Doty P. A Light Scattering Investigation of Cellulose Acetate1. J. Am. Chem. Soc. 1946; 68(2), 159–167.. While traditional unit operations enable target biologic purification by either size or affinity interaction, the IsoTag™AAV technology consolidates both mechanisms into one simple, elegant process.
Technology
The IsoTag™AAV technology combines the principles of affinity capture with liquid-liquid phase separation. This is achieved with a proprietary fusion protein comprising an AAV-specific affinity ligand and a stimulus-responsive biopolymer fused together at the gene level and produced recombinantly as a single fusion protein. Stimulus-responsive polymers are a class of macromolecules that undergo liquid phase separation with an external environmental trigger, such as temperature, salt, or pH [14]Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999; 17(11), 1112–1115.. Thermally-responsive polymers include many families of materials that undergo a phase transition in response to changes in temperature and have been studied for their use in a variety of biomedical applications such as drug delivery and tissue engineering [15]Bhattacharyya J, Bellucci JJ, Weitzhandler I et al. paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat. Commun. 2015,6, 7939. [16]Luginbuhl KM, Schaal JL, Umstead B et al. One-week glucose control via zero-order release kinetics from an injectable depot of glucagon-like peptide-1 fused to a thermosensitive biopolymer. Nat. Biomed. Eng. 2017; 1. [17]MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 2009; 8(12), 993–999. [18]Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010,62(15), 1479–1485. [19]Municoy S, Álvarez Echazú MI, Antezana PE et al. Stimuli-responsive materials for tissue engineering and drug delivery. International Journal of Molecular Sciences 2020; 21(13), 4724.. With scalable manufacturing of AAV in mind, the IsoTag™AAV reagent’s biopolymer component has been engineered to undergo phase transition at ambient temperature with manipulation of salt concentration. The phase separated IsoTag™AAV reagent is fully reversible, returning to a small, fully soluble protein by lowering the temperature or lowering the salt concentration of the solution.
IsoTag™AAV reagents offer an innovative approach to AAV purification. Highly specific affinity for AAV can be toggled on and off by manipulating solution conditions, such as pH and salt, that affect the ligand’s binding affinity (Kd) to the AAV capsid. The effective size of the virus-IsoTag™ complex can be adjusted in situ by orders of magnitude (22 nm to >1000 nm) through the reversible phase separation phenomenon and its bound or unbound state.
Purification of AAV with this reagent is depicted in the schematics of Figure 1 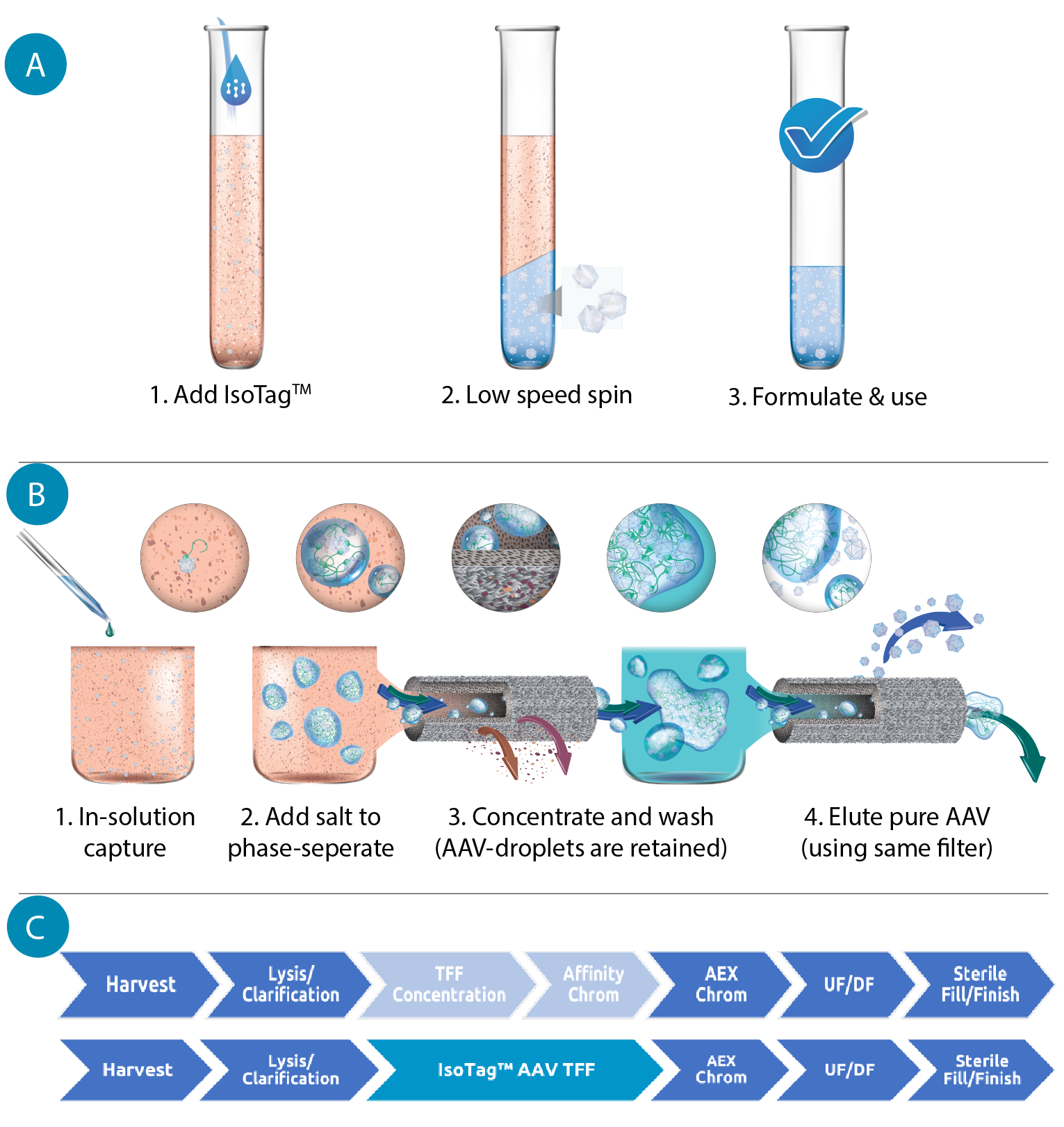 (A) Schematic of the IsoTag™️AAV process with density-mediated capture. Using centrifugation, the AAV is scavenged from the harvest media. (B) Schematic of the IsoTag™️AAV process with size-mediated capture. Using microfiltration, the AAV can be captured and concentrated in the retentate of a TFF hollow fiber. Using buffer exchanges, the AAV can be purified in the retentate and then eluted from the IsoTag™️ reagent and collected in the permeate. (C) Flow chart of steps in a traditional AAV downstream process compared to a downstream process using IsoTag™️AAV technology. IsoTag™️AAV streamlines purification, consolidating two unit operations into one step that is more efficient. . When added to cell culture harvests, the affinity ligand portion of the IsoTag™AAV reagent specifically binds the AAV capsids. Salt is then added to the solution, triggering lower critical solution temperature (LCST) phase separation. In this state, the IsoTag™AAV forms large, micron-scale coacervates containing the bound AAV, sequestered and protected from contaminants. The AAV-containing coacervates can be separated from the bulk harvest through centrifugation by pelleting the dense, protein-rich droplets, or through microfiltration (TFF) by retaining the large droplets while harvest media and contaminants are eliminated as waste through the filter permeate. The pelleted or retained capsid-containing IsoTag™AAV droplets can then be resolubilized by lowering the solution’s temperature or salinity. The AAV capsid is then dissociated from the IsoTag™AAV reagent by lowering the pH of the solution to disrupt the binding of the affinity ligand. The phase transition is once again triggered by the addition of salt; however, in this elution environment, the unbound AAV remains in solution and is not pulled into the coacervates. Now unbound, purified AAV can be separated from the IsoTag™AAV droplets using the same methods as the capture step.
(A) Schematic of the IsoTag™️AAV process with density-mediated capture. Using centrifugation, the AAV is scavenged from the harvest media. (B) Schematic of the IsoTag™️AAV process with size-mediated capture. Using microfiltration, the AAV can be captured and concentrated in the retentate of a TFF hollow fiber. Using buffer exchanges, the AAV can be purified in the retentate and then eluted from the IsoTag™️ reagent and collected in the permeate. (C) Flow chart of steps in a traditional AAV downstream process compared to a downstream process using IsoTag™️AAV technology. IsoTag™️AAV streamlines purification, consolidating two unit operations into one step that is more efficient. . When added to cell culture harvests, the affinity ligand portion of the IsoTag™AAV reagent specifically binds the AAV capsids. Salt is then added to the solution, triggering lower critical solution temperature (LCST) phase separation. In this state, the IsoTag™AAV forms large, micron-scale coacervates containing the bound AAV, sequestered and protected from contaminants. The AAV-containing coacervates can be separated from the bulk harvest through centrifugation by pelleting the dense, protein-rich droplets, or through microfiltration (TFF) by retaining the large droplets while harvest media and contaminants are eliminated as waste through the filter permeate. The pelleted or retained capsid-containing IsoTag™AAV droplets can then be resolubilized by lowering the solution’s temperature or salinity. The AAV capsid is then dissociated from the IsoTag™AAV reagent by lowering the pH of the solution to disrupt the binding of the affinity ligand. The phase transition is once again triggered by the addition of salt; however, in this elution environment, the unbound AAV remains in solution and is not pulled into the coacervates. Now unbound, purified AAV can be separated from the IsoTag™AAV droplets using the same methods as the capture step.
The IsoTag™AAV approach to purification offers many benefits compared to existing affinity resins. The technology combines two unit operations typically present in downstream AAV purification – a TFF step for concentration and buffer exchange plus an affinity chromatography step. Our process also eliminates ancillary pain points associated with the current downstream structure, such as column packing and associated failures, long loading times, and scalability of TFF unit operations. In total, the IsoTag™AAV technology saves time and costs by improving total yield through unit operation reduction. Importantly, the IsoTag™AAV TFF process is based on harvest volume, not titer, and can therefore be scaled up linearly by simply increasing the filter surface area proportionally to feed volume. This allows for simple execution and scale-up across a variety of pipeline molecules. It also makes the process independent of upstream performance, allowing for significant flexibility while also accommodating improvements from upstream without changes to the downstream process.
IsoTag™AAV is a recombinant protein reagent that can capture AAV, followed by sequestration – and separation from other contaminants – in stable, surfactant-free droplets that are highly enriched in virus. This eliminates the requirement of a solid scaffold (e.g. resins, beads, membranes, monoliths) entirely, creating two important consequences: first, it eliminates the chemical conjugation step that is used to manufacture an affinity matrix, and second, it completely eliminates the mass transport factors that are intrinsic and limiting to heterogeneous separation processes such as affinity chromatography. Although technologies, such as membranes, fibers, and monoliths, avoid the mass transfer limitations of traditional packed beds, their adoption has been largely limited to flow through mode operations with ion exchange rather than affinity-mediated capture-elute mode.
The IsoTag™AAV reagent can be produced in a single synthesis step in E. coli for fast, straightforward manufacturing. The use of this expression system allows us to leverage existing recombinant protein manufacturing infrastructure for simple production at various scales and various quality grades from research to GMP. As the recombinant protein reagent itself is <50 kDa, it can also be supplied as a fully sterile product in aqueous buffer, thereby eliminating the need for hazardous storage chemicals and opening up opportunities for fully closed processing.
The IsoTag™AAV process was first validated with the centrifugation format at small scale (1mL) to screen feed stream compatibility and to optimize process buffers. During this evaluation phase we determined basic operation parameters for the IsoTag™AAV process and confirmed broad serotype compatibility to AAV vectors of serotype 1, 2, 6, 8, 9, rH10 & PHP.B ( Figure 2 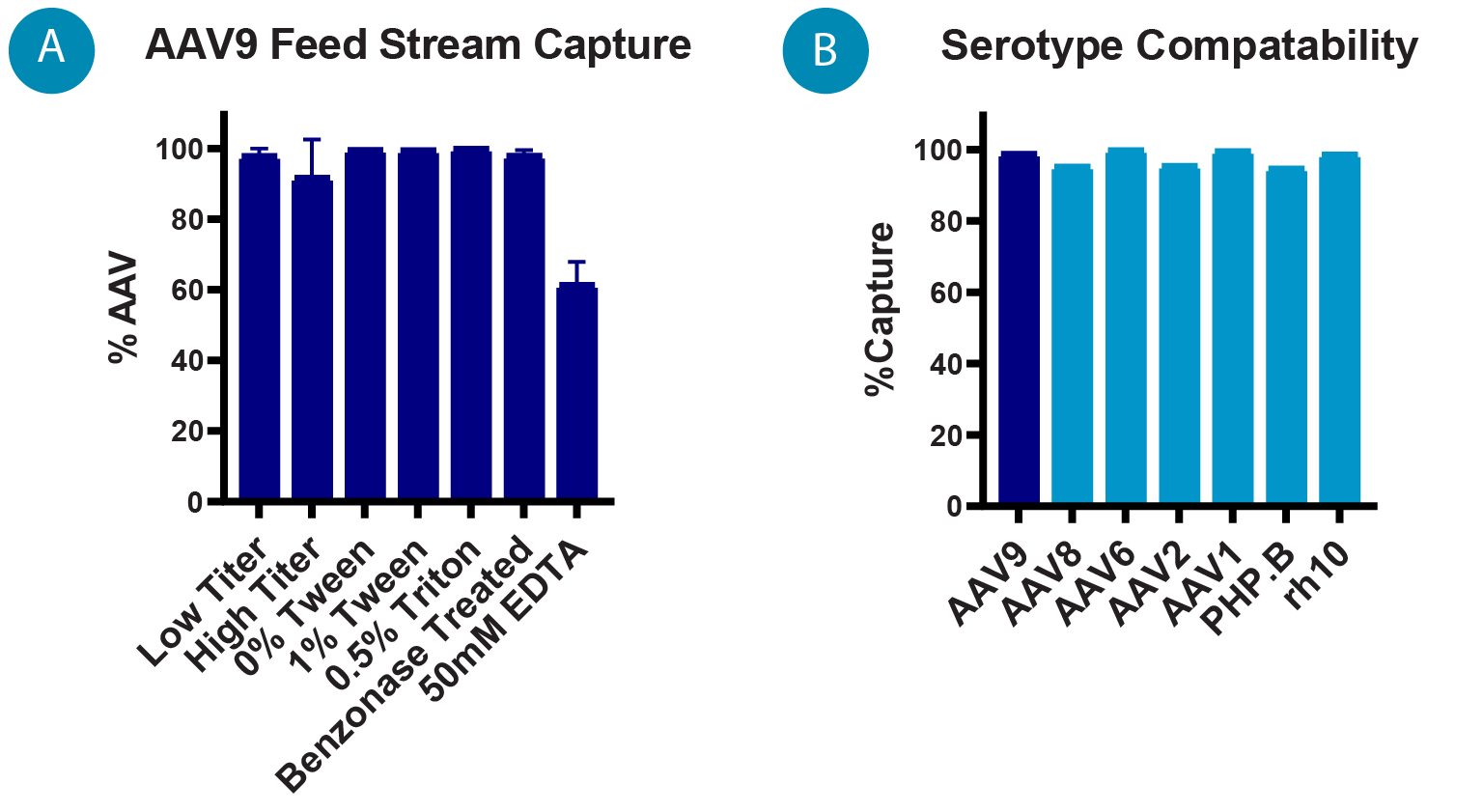 (A) Percentage of AAV9 captured by IsoTag™️AAV centrifugation process for various feed streams (n = 3). (B) Percent capture of different AAV serotypes using small-scale centrifugation process. Error bars represent standard deviation of the mean. B). The IsoTag™AAV capture and elution was then transitioned to a small-scale TFF process (0.2 L) using clarified AAV9 lysate. Next, the scalability of the process was evaluated with 1 L and 8 L batch volumes by increasing TFF filter area, but keeping all other parameters identical, including volumetric loading, shear rate, and flux. Finally, the performance of this process was evaluated by a leading gene therapy company by splitting a batch of cell culture harvest and running IsoTag™AAV TFF purification in parallel with their industry standard process. The two processes were compared head-to-head for productivity, yield, and quality. Based on these results, a detailed model was created to explore the value of IsoTag™AAV if it were implemented in larger scale clinical campaigns.
(A) Percentage of AAV9 captured by IsoTag™️AAV centrifugation process for various feed streams (n = 3). (B) Percent capture of different AAV serotypes using small-scale centrifugation process. Error bars represent standard deviation of the mean. B). The IsoTag™AAV capture and elution was then transitioned to a small-scale TFF process (0.2 L) using clarified AAV9 lysate. Next, the scalability of the process was evaluated with 1 L and 8 L batch volumes by increasing TFF filter area, but keeping all other parameters identical, including volumetric loading, shear rate, and flux. Finally, the performance of this process was evaluated by a leading gene therapy company by splitting a batch of cell culture harvest and running IsoTag™AAV TFF purification in parallel with their industry standard process. The two processes were compared head-to-head for productivity, yield, and quality. Based on these results, a detailed model was created to explore the value of IsoTag™AAV if it were implemented in larger scale clinical campaigns.
Results
IsoTag™AAV reagent was successfully cloned, expressed in E. coli in a shake flask format and purified. After successful development of expression and purification methods in the shake flask format, the manufacturing was scaled to 2 L and 10 L bioreactors. Reagent purity was confirmed with SDS-PAGE ( Figure 3 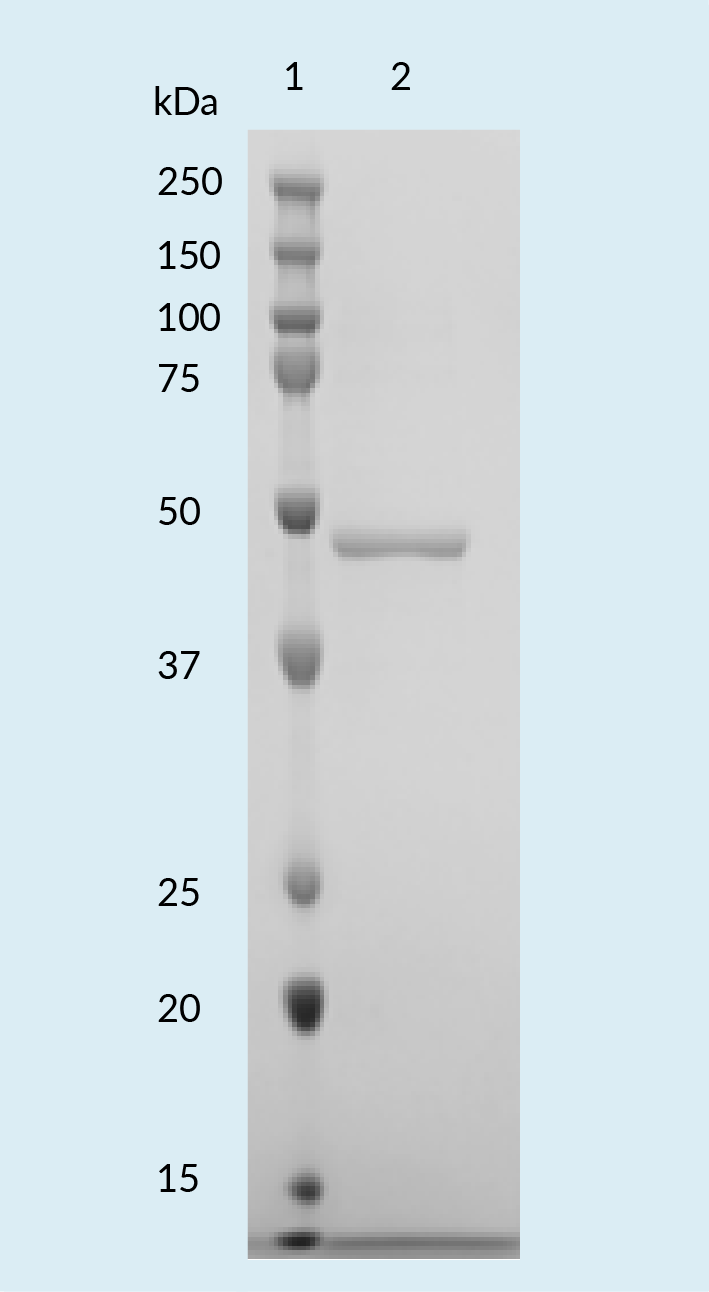 SDS-PAGE of IsoTag™️AAV reagent expressed and purified from E. coli. ).
SDS-PAGE of IsoTag™️AAV reagent expressed and purified from E. coli. ).
Initial proof-of-concept for AAV9 capture and elution was performed in a small-scale centrifugation format. The capture for each sample was determined by comparing the AAV titer of the capture supernatant to the titer of the starting cell culture harvest to determine the percentage of AAV retained in the capture pellet. The elution percentage was determined by comparing the titer of the neutralized, eluted AAV to the titer of the starting cell culture harvest.
Repeated centrifugation testing with IsoTag™AAV demonstrated ~95% capture of the AAV9 present in cell culture harvest and resulted in an elution yield of ~80% ( Figure 4 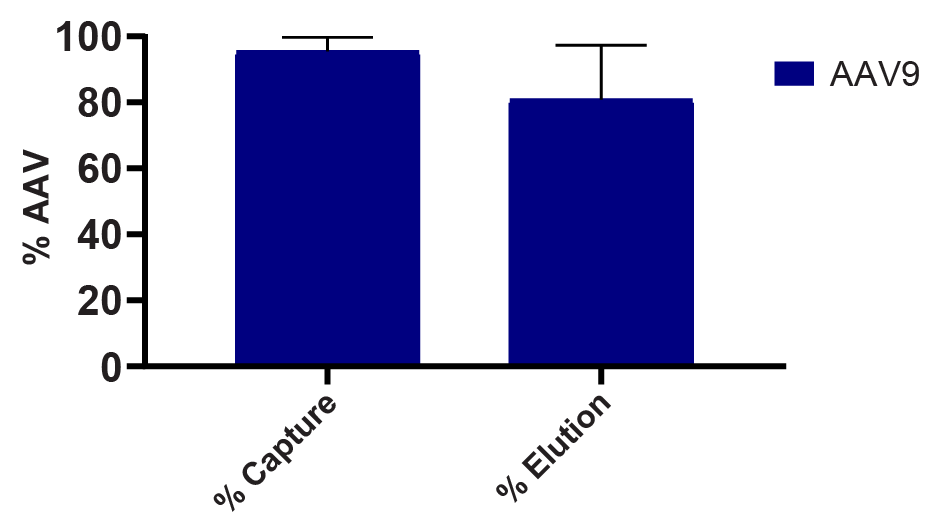 Percentage of AAV9 harvest captured and eluted using IsoTag™AAV centrifugation method by qPCR. n = 3, error bars represent standard deviation of the mean. ). The same small-scale capture format was used to screen capture and elution from different AAV9 feed streams to determine compatibility of IsoTag™AAV with harvests of various titers, as well as samples that had undergone various lysis and clarification protocols (Figure 2A).
Percentage of AAV9 harvest captured and eluted using IsoTag™AAV centrifugation method by qPCR. n = 3, error bars represent standard deviation of the mean. ). The same small-scale capture format was used to screen capture and elution from different AAV9 feed streams to determine compatibility of IsoTag™AAV with harvests of various titers, as well as samples that had undergone various lysis and clarification protocols (Figure 2A).
In the centrifugation format, IsoTag™AAV capture and elution was found to be compatible with starting titers ranging from 4 x 109 to 1 x 1013 vc/mL (Figure 2A). IsoTag™AAV performance was also evaluated with commonly used lysis buffer components and was found to be compatible with up to 1% Tween and 0.5% Triton X-100. However, addition of EDTA to the lysis buffer negatively impacted capture by IsoTag™AAV. In the centrifugation format, IsoTag™AAV capture was found to be compatible with material treated with benzonase concentrations ranging from 5 U/mL to 50 U/mL as well as harvest supernatant that had not undergone any nuclease treatment.
Based on these data, we expect optimization efforts for IsoTag™ capture conditions to be minimal, and any wash and elution buffer optimization to follow similar design of experiment models currently employed for chromatographic processes today to maximize yield and contaminant removal for each molecule.
The IsoTag™AAV capture and elution process was transitioned into a more scalable TFF format using Spectrum hollow fiber filters and a KrosFlo KR2i system (Repligen Corporation). Through evaluation of various hollow fiber options, filters with a 0.2-micron pore size, 0.5 mm lumen, and 20 cm effective length were found to yield efficient and reproducible capture. Although testing has not been exhaustive, initial screening experiments demonstrate that flat sheet formats also appear to be compatible, though may require some additional optimization to match the current hollow fiber performance detailed herein.
Additional TFF runs were completed to establish a baseline protocol, which employs 6000 sec-1 shear rate, ~50 LMH flux, and 6 wash buffer diavolumes (DVs). Evaluation of elution conditions identified a bulk addition step of cold elution buffer (100 mM glycine, pH 3.0) with the permeate valve closed. This resolubilizes the IsoTag™AAV droplets and releases the captured AAV, after which MgCl2 is added to 0.6 M concentration to re-trigger the phase transition. A 10X concentration of the retentate followed by 8 elution DVs (100 mM glycine pH 3.0, 0.6 M MgCl2) provided the highest yields. The total processing time for capture, purification, and elution was ~4 hours. Contaminant removal throughout the TFF process resulted in highly pure AAV that was recovered during elution with minimal loss during the flow-through and wash steps ( Figure 5 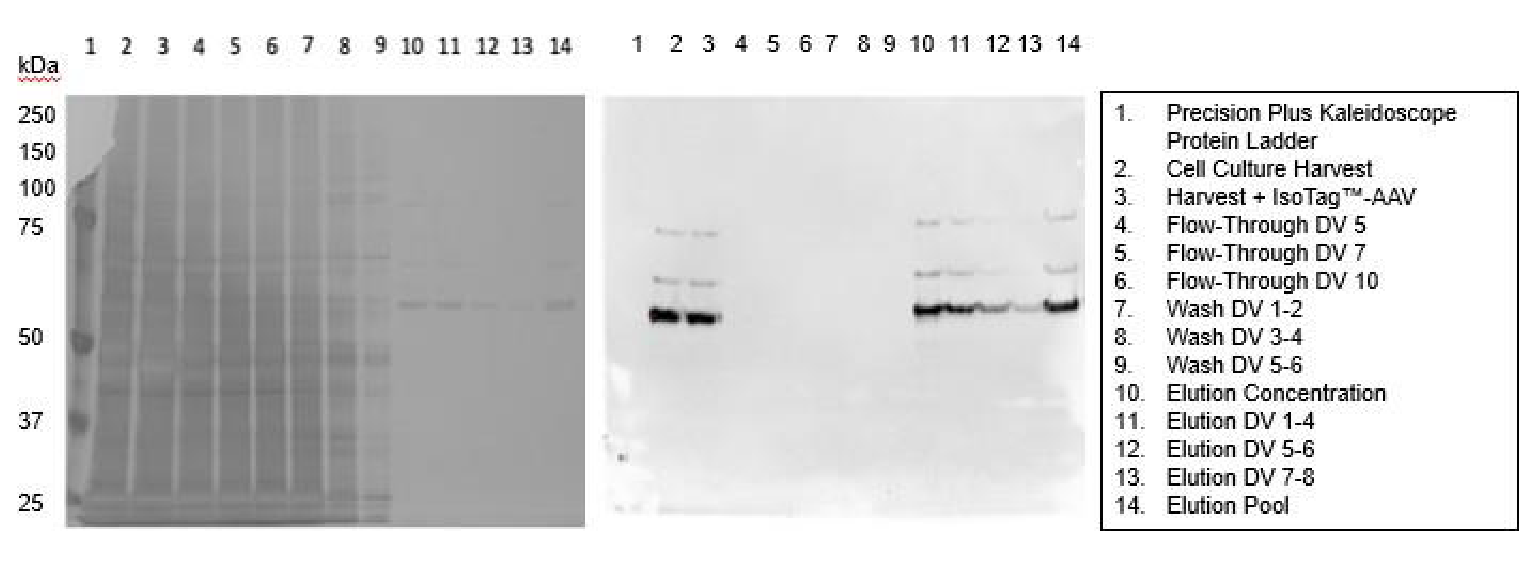 SDS-PAGE gels of fractions taken throughout the TFF process to show: Left: purity of samples with silver staining and Right: presence of AAV capsids with an anti-AAV (VP1, VP2, VP3) immunoblot. ).
SDS-PAGE gels of fractions taken throughout the TFF process to show: Left: purity of samples with silver staining and Right: presence of AAV capsids with an anti-AAV (VP1, VP2, VP3) immunoblot. ).
The IsoTag™AAV TFF process was scaled up from 0.2 L to 1 L and 8 L scales by linearly and proportionately increasing the filter area to keep volumetric loading of the feed constant. The permeate controlled flux was also kept constant at all volumes. The resulting runs had nearly identical processing times of ~3.5 hours.
As the process was scaled up with the same harvest material, the TMP profile improved, indicating that overall performance is improved as the process scales ( Figure 6 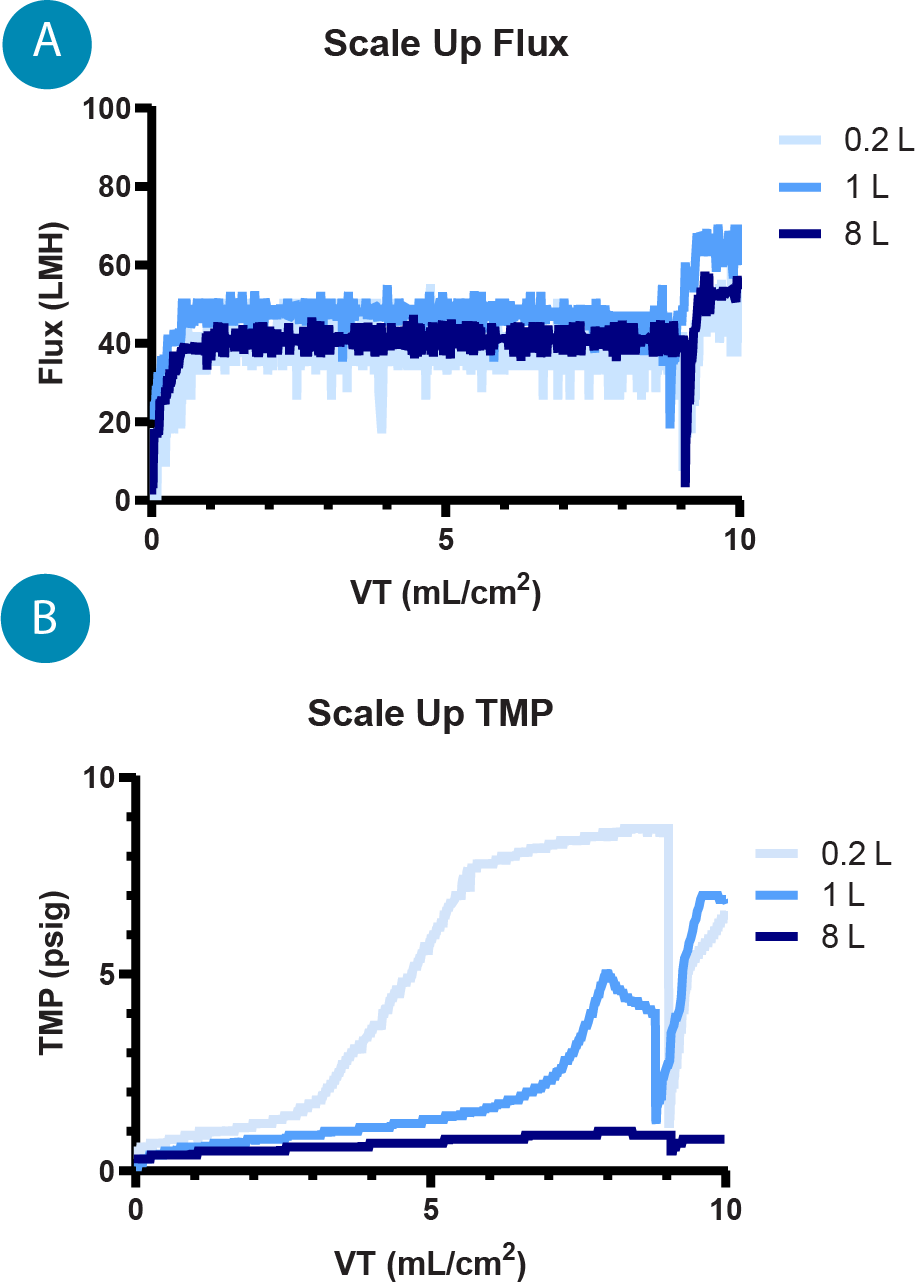 (A) Flux profiles for 0.2, 1, and 8 L TFF runs, the flux is controlled by the permeate pump to maintain a constant 40–50 LMH. (B) TMP profiles for 0.2 L, 1 L, and 8 L TFF runs where the TMP, measured throughout the run, is an indication of gel layer formation and filter performance. ), hypothesized to be due to better flow paths and fluid dynamics across larger fiber bundles. The capture by IsoTag™AAV was consistent at each scale with average captures of 96.6% and 98.2% by qPCR and total capsid ELISA, respectively. The total elution yield for the process across each scale was 75.3% and 74.7% by qPCR and ELISA, respectively ( Figure 7
(A) Flux profiles for 0.2, 1, and 8 L TFF runs, the flux is controlled by the permeate pump to maintain a constant 40–50 LMH. (B) TMP profiles for 0.2 L, 1 L, and 8 L TFF runs where the TMP, measured throughout the run, is an indication of gel layer formation and filter performance. ), hypothesized to be due to better flow paths and fluid dynamics across larger fiber bundles. The capture by IsoTag™AAV was consistent at each scale with average captures of 96.6% and 98.2% by qPCR and total capsid ELISA, respectively. The total elution yield for the process across each scale was 75.3% and 74.7% by qPCR and ELISA, respectively ( Figure 7 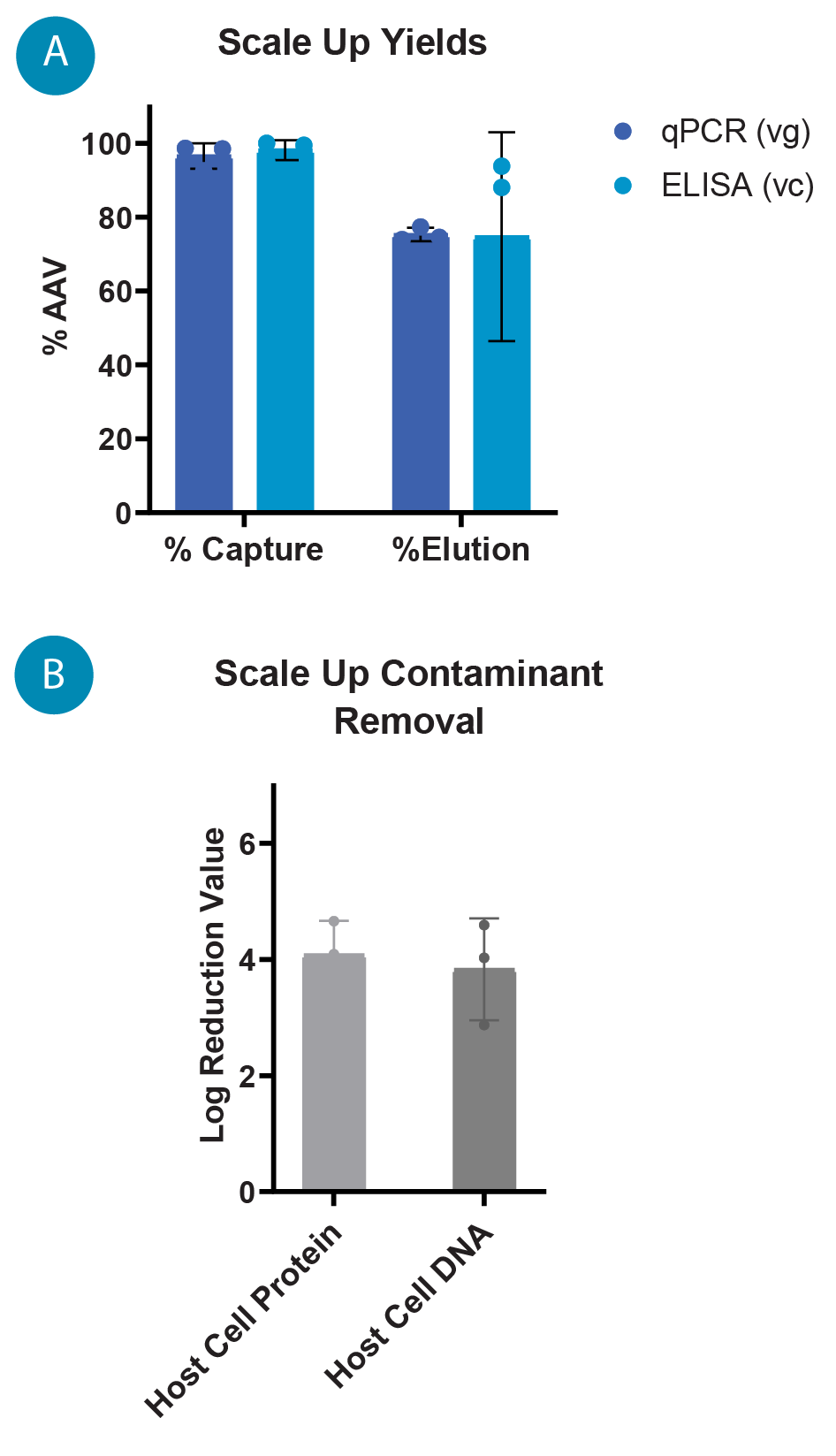 (A) AAV9 capture and elution percentages with IsoTag™️AAV TFF across 0.2 L, 1 L and 8 L scales by qPCR (vg) and total capsid ELISA (cp). Percentages are based on total AAV9 present in cell culture harvest prior to TFF. (B) Log reduction values (LRVs) for host cell protein and host cell DNA contaminants using the IsoTag™️AAV TFF process at 0.2 L, 1 L and 8 L scales. A). It is also worth noting that the majority of the AAV that was not recovered during elution is found in the retentate. Losses to the permeate during capture and wash steps are approximately 5%, and additional yields of AAV may be achieved by further optimization of elution process parameters.
(A) AAV9 capture and elution percentages with IsoTag™️AAV TFF across 0.2 L, 1 L and 8 L scales by qPCR (vg) and total capsid ELISA (cp). Percentages are based on total AAV9 present in cell culture harvest prior to TFF. (B) Log reduction values (LRVs) for host cell protein and host cell DNA contaminants using the IsoTag™️AAV TFF process at 0.2 L, 1 L and 8 L scales. A). It is also worth noting that the majority of the AAV that was not recovered during elution is found in the retentate. Losses to the permeate during capture and wash steps are approximately 5%, and additional yields of AAV may be achieved by further optimization of elution process parameters.
Log reduction values (LRVs) for host cell protein (HCP) and host cell DNA (HCD) were quantified using a HEK HCP ELISA (Cygnus) and Quant-iT PicoGreen dsDNA Assay (Thermo Scientific), respectively. HCP and HCD contaminants in the clarified lysate feed are significantly reduced by IsoTag™AAV at all scales, with LRVs averaging 4.1 for HCP and 3.8 for HCD (Figure 7B).
Next, to externally validate the technology, the performance of IsoTag™AAV was evaluated in a collaborative effort with Capsida Biotherapeutics, a gene therapy company that is engineering novel capsids for improved gene therapies. Capsida compared Isolere’s baseline process to a standard AAV9-specific affinity chromatography method (Table 1). TFF-based purification with IsoTag™AAV resulted in 80.3% yield by ddPCR and ~100% yield by total capsid ELISA. By comparison, the affinity chromatography process yielded 61.2% by ddPCR and 62.6% by total capsid ELISA. As assessed by CE-SDS, the purity of AAV by Isolere’s process was 99% whereas the AAV purity by the standard chromatography process was only 88%. Dynamic light scattering showed no appreciable differences in final product size or aggregation. Finally, AAV eluted from IsoTag™AAV compared to AAV purified by affinity chromatography had superior clearance of HCP and HCD. These results serve as external validation of the IsoTag™AAV technology, showing similar results to those generated internally, as well as promising performance when compared to an industry leading process.
| Table 1. Summary of key metrics from a head-to-head study conducted at Capsida Biotherapeutics, Inc. comparing AAV purification with IsoTag™AAV TFF versus TFF plus affinity chromatography. | ||
| Key metrics | IsoTag™AAV | Affinity chromatography |
| Process time (h) | 3 | 5 |
| Yield (vg %) | 80% | 61% |
| Yield (cp %) | 118% | 63% |
| Purity (CE-SDS %) | 99% | 88% |
| Diam (nm) / PDI / Peak % | 28 nm / 0.05 / 100% | 27 nm / 0.05/ 100% |
| TCID 50 (vg/IU) | 3.6 x 103 | 9.3 x 103 |
| HCP (LRV/dose) | 4.9 | 4.1 |
| HCD (LRV/dose) | 4.3 | 3.7 |
The promising results at 1 L scale with Capsida prompted the development of a highly sophisticated model in collaboration with Biopharm Services to better understand the potential impact of the IsoTag™AAV technology at clinical and commercial scales. Two process models were developed to holistically understand differences in end-to-end production of AAV with an industry standard process (TFF plus affinity chromatography) compared to one where both steps are replaced by IsoTag™AAV. Each process was modeled using a 200 L reactor volume, assuming a 12-month single bioreactor campaign, 1e11 vg/mL titer, 10% full capsid percentage, 1e17 cp/L resin capacity, a 1 x 1013 vg dose, and single use technology for all process and support equipment.
The modeling shows that IsoTag™AAV leads to ~28% lower cost of goods per dose of AAV purified. This is primarily driven by the increased yield of the IsoTag™AAV process compared to the affinity process and reduced time of operation, leading to lower labor costs ( Figure 8 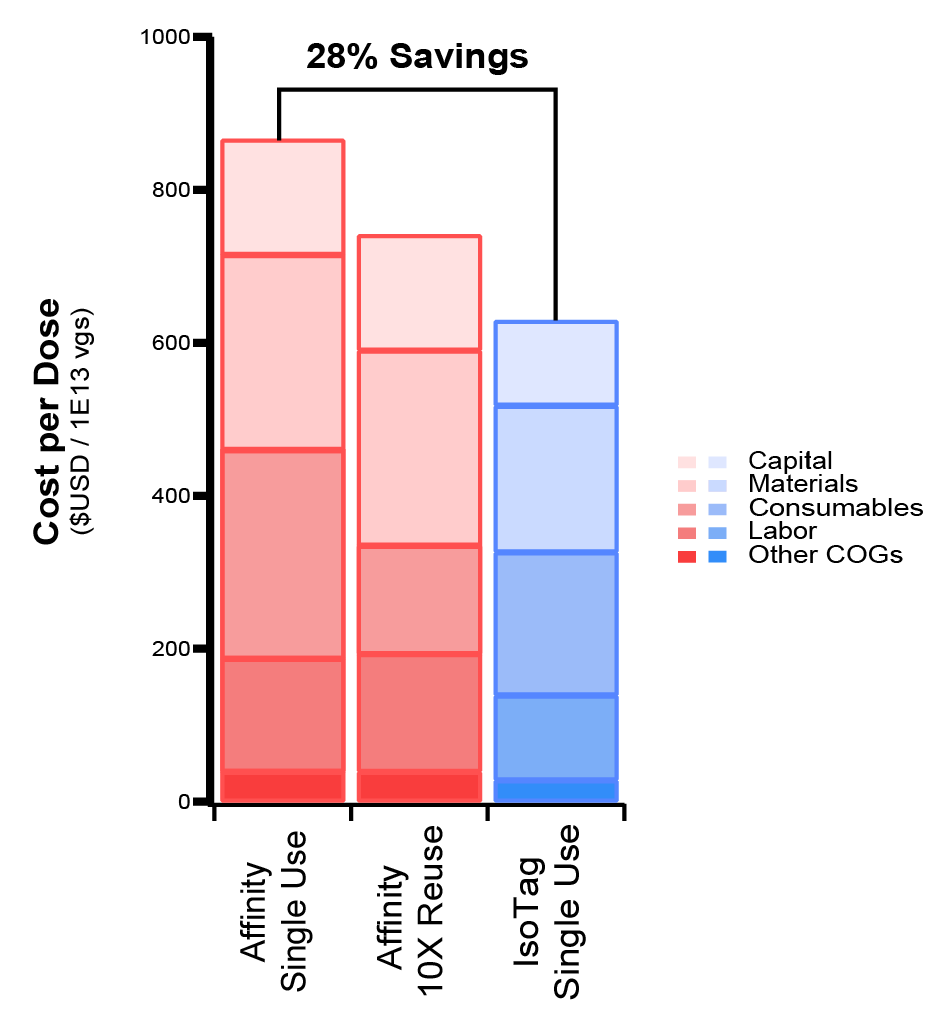 Cost breakdown per dose when implementing IsoTag™️AAV process compared to an affinity-based process modeled after the Capsida comparison study. The current IsoTag™️AAV process could improve yield and lower cost per dose by >25% and this new process remains COGs competitive as a single use technology, even against 10X reuses of the affinity resin. ). Even assuming 10X reuse of an affinity resin, which could be expected for commercial manufacturing, the IsoTag™AAV process is still cost competitive with single-use implementation, which serves to further streamline operations and reduce the risk and operational complexity that is incurred by resin reuse. Further cost reduction potential of the IsoTag™AAV technology through regeneration and reuse testing and validation is ongoing.
Cost breakdown per dose when implementing IsoTag™️AAV process compared to an affinity-based process modeled after the Capsida comparison study. The current IsoTag™️AAV process could improve yield and lower cost per dose by >25% and this new process remains COGs competitive as a single use technology, even against 10X reuses of the affinity resin. ). Even assuming 10X reuse of an affinity resin, which could be expected for commercial manufacturing, the IsoTag™AAV process is still cost competitive with single-use implementation, which serves to further streamline operations and reduce the risk and operational complexity that is incurred by resin reuse. Further cost reduction potential of the IsoTag™AAV technology through regeneration and reuse testing and validation is ongoing.
Conclusions
As demonstrated herein, IsoTag™AAV is a promising technology for improving and streamlining the manufacturing of AAV. It has the potential to accelerate the development of therapies by reducing the time spent optimizing and scaling a process. Furthermore, this technology could profoundly impact the commercial feasibility of therapeutics by improving overall productivity per batch and harmonizing scale up from the bench to the clinic and beyond. Compared to industry-leading methods, IsoTag™AAV provided a more streamlined process with higher yields, higher purity, and equivalent or better contaminant removal. As a process that is performed on the basis of volume rather than titer, IsoTag™AAV has the ability to accommodate major improvements in upstream virus production and to also accommodate upstream changes between batches or pipeline molecules with ease. While AAV is the first application of this technology, IsoTag™ has broad potential to become a highly enabling, first-in-class manufacturing solution for advanced therapeutics. Isolere is actively developing reagents for lentivirus and mRNA, among other complex biologic modalities.
Methods
IsoTag™AAV production
The gene encoding the IsoTag™AAV fusion protein was cloned using recursive directional ligation by plasmid reconstruction (PRe-RDL), as described by [17]MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 2009; 8(12), 993–999.. The plasmid containing the gene of interest was then transformed into E. coli competent cells for expression and fermentation. Fusion protein was produced in shake flasks and purified to >90% purity for testing. 10–25X stock solutions of the reagent were made using both Beer’s law calculation from their absorbance at 280 nm, as well as precise weights of lyophilized protein powders.
Centrifugation capture screening
Small volume screening of IsoTag™AAV capture was conducted using a centrifugation format. These experiments were performed using 1 mL of cell culture harvest in triplicate for the following serotypes: AAV1, AAV2, AAV6, AAV8, AAV9, AAV PHP.B and AAV rh10. IsoTag™AAV was added to each 1 mL aliquot to a 1X concentration to bind the AAV. A 5 M sodium chloride stock was then added to a final concentration of 0.6 M, with mixing, to trigger the phase separation of the biopolymer. The samples were centrifuged at 13 krpm for 10 min at room temperature to pellet the AAV-containing IsoTag™ coacervates. The supernatant, containing soluble contaminants from the cell culture harvest and any uncaptured AAV, was removed and saved for analysis by ITR2 qPCR and total capsid ELISA. Elution buffer (100 mM Glycine, pH 3.0) was added to the pellets and left rotating in a cold room for ~1 h to resolubilize the IsoTag™AAV by reversing the phase transition, while the low pH buffer dissociates the AAV from the IsoTag™. Magnesium chloride was then added to a concentration of 0.6 M to trigger the phase separation of the IsoTag™. After a final centrifugation step at 13 krpm for 10 min at room temperature, the eluted AAV remains in the soluble fraction. This pure, eluted AAV was transferred to a new tube, neutralized with 1 M Tris pH 7.5, and saved for analysis.
AAV lysis & clarification
Harvest containing rAAV9 was produced by culturing HEK cells in suspension with a triple plasmid transfection method. Clarified lysate was used for the process development and scale-up of the IsoTag™AAV TFF purification process. Frozen AAV9 harvest material was thawed and lysed using 0.5% Tween-20 and incubating for 15–30 min. The lysed harvest material was then nuclease treated by adding 2 mM MgCl2 and 5 U/mL Benzonase and incubating at 37 °C for 1–2 h. Pluronic acid was then added to the lysate to a concentration of 0.01% to prevent AAV adsorption to tubing, containers and filter surfaces, and the lysate was then clarified using a MilliPore MilliStak D0SP uPod depth filter. Sodium chloride was then added to the clarified lysate to a concentration of 0.6 M and the material was filtered with a 0.2 µm bottle-top filter.
IsoTag™AAV TFF process development & scale up
The IsoTag™AAV TFF process was developed on a Repligen KrosFlo Kr2i system using Spectrum Hollow Fiber filters set up as shown in Figure 9 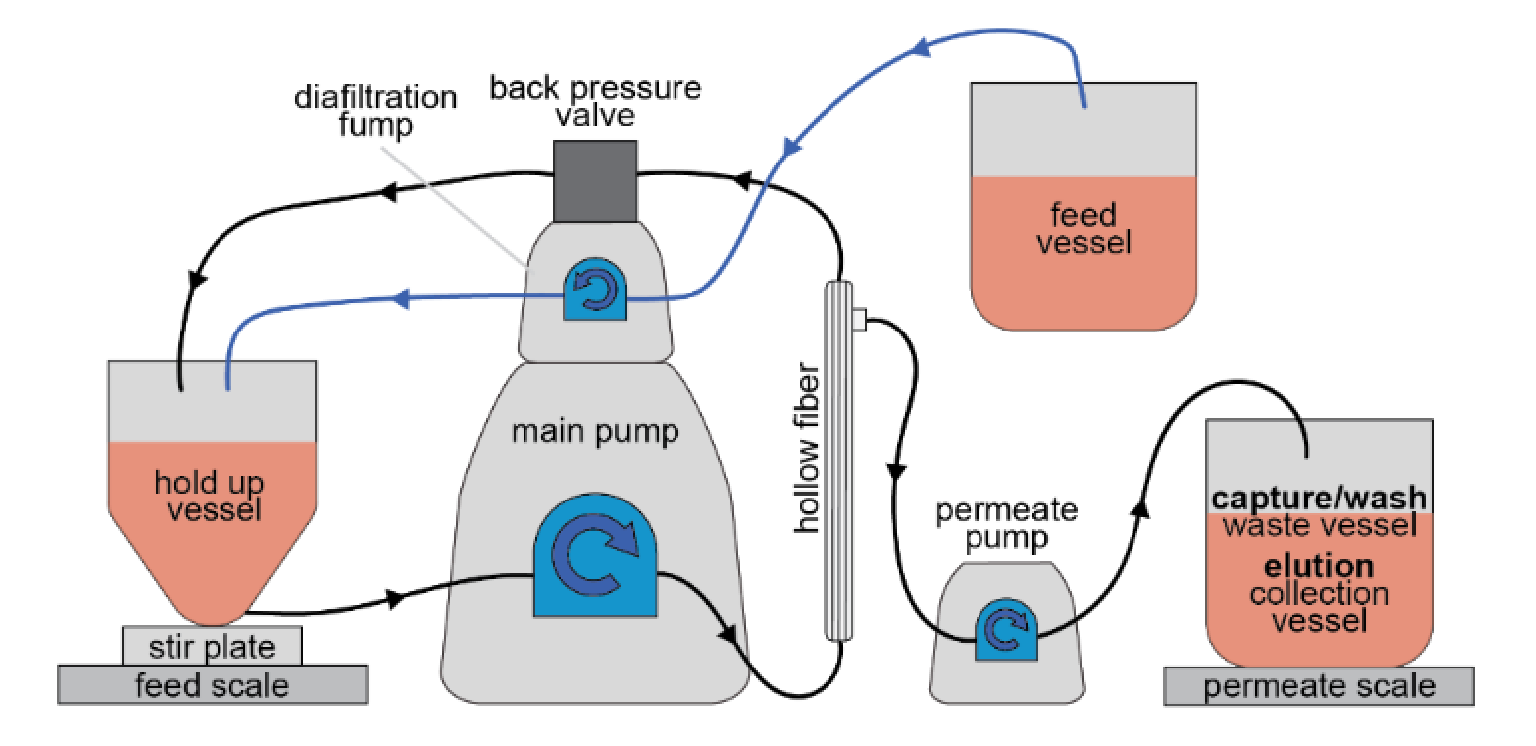 Schematic for TFF system setup for IsoTag™️AAV purification. The setup is configured for permeate control mode with a hollow fiber filter and a stirred, bottom-fed retentate vessel. . The process was run using a PendoTECH lab scale process vessel with a conical bottom and low point drain with a stir bar. The hold-up vessel was placed on a stir-plate on the feed scale of the Kr2i system. An auxiliary pump was used to run the system in permeate control mode at a set permeate rate while another auxiliary pump was used to feed bulk harvest material into the hold-up vessel at an equal rate to maintain a constant hold-up volume. A back pressure valve was used to maintain a constant pressure of 10 PSI on the retentate line. The cross-flow rate of the system was set to maintain a 6000 s-1 shear rate and the permeate rate was set to maintain a 50–60 LMH flux rate.
Schematic for TFF system setup for IsoTag™️AAV purification. The setup is configured for permeate control mode with a hollow fiber filter and a stirred, bottom-fed retentate vessel. . The process was run using a PendoTECH lab scale process vessel with a conical bottom and low point drain with a stir bar. The hold-up vessel was placed on a stir-plate on the feed scale of the Kr2i system. An auxiliary pump was used to run the system in permeate control mode at a set permeate rate while another auxiliary pump was used to feed bulk harvest material into the hold-up vessel at an equal rate to maintain a constant hold-up volume. A back pressure valve was used to maintain a constant pressure of 10 PSI on the retentate line. The cross-flow rate of the system was set to maintain a 6000 s-1 shear rate and the permeate rate was set to maintain a 50–60 LMH flux rate.
For the 0.2 L process scale, a Spectrum C02-P20U-05-N (0.2 µm pore size, 0.5 mm ID, 23 cm total length, 28 cm2 surface area) hollow fiber filter was used with size 16 tubing for the recirculation loop and size 13 tubing for the permeate line. The system was flushed with a 0.6 M NaCl buffer prior to filtration. A 25x concentrate solution of IsoTag™AAV was added to 10X final concentration in 20 mL of the clarified lysate, prepared as described above. IsoTag™AAV was added to a 1X concentration to the remaining lysate volume. The 20 mL of lysate combined with 10X IsoTag™AAV was added to the hold-up vessel, while the bulk harvest was placed in a feed reservoir connected to the hold-up vessel with size 16 tubing and an auxiliary pump. The system was run in a concentration-diafiltration (C-D) mode with a concentration factor (CF) of 1 (to maintain a constant hold-up volume) and 16 diafiltration volumes (DV) to process the clarified lysate in a continuous fed batch setup. The cross-flow rate was set to 45 mL/min and, once the backpressure valve had achieved a constant 10 PSI pressure on the retentate line, the permeate flow was started at a rate of 0.3 mL/min and slowly increased until a flux of 50–60 LMH was achieved. Once the entire volume of clarified lysate had been added to the hold-up vessel via the diafiltration auxiliary pump, a wash buffer containing 20 mM Tris pH 7.5, 0.6 M NaCl, 0.01% pluronic acid was added to the feed reservoir. The diafiltration process was continued until 6 DV of wash buffer had passed through the permeate line. Samples were taken from the capture flow-through and wash fractions for analytics, the remaining capture and wash permeate was discarded.
Following the capture and wash process, 80 mL of elution buffer (100 mM Glycine pH 3.0, 0.01% pluronic) that had been chilled on ice was added directly to the hold-up vessel containing the captured AAV and IsoTag™. The addition of the cold, low-salt elution buffer resolubilized the IsoTag™AAV, while the low pH dissociated the AAV from the ligand. The system was set to recirculate at 45 mL/min with the permeate line closed to allow thorough and complete solubilization and elution of the IsoTag™AAV. After 5 min of recirculation, 14 mL of 5 M MgCl2 was added to the hold-up vessel to a final concentration of 0.6 M to trigger the phase separation of the IsoTag™ once more and the solution was allowed to recirculate for an additional 10 min. The permeate line was then reopened and the system was run in C-D mode with a CF of 10 and 8 DVs.
During the concentration step, the hold-up volume was concentrated 10X to a final volume of ~11 mL while unbound AAV in solution was passed through the permeate line. Following the elution concentration, 8 DV of elution buffer containing salt (100 mM Glycine pH 3, 0.6 M MgCl2, 0.01% pluronic) was used to wash remaining free AAV into the permeate. The permeated elution material was neutralized by adding a volume of neutralization buffer (1 M Tris, pH 7.5) equal to 10% of the fraction volume. Neutralized elution fractions were collected and the bulk eluted material was pooled. The elution pool was concentrated and buffer exchanged into DPBS, 0.01% pluronic using Pierce PES Protein Concentrator tubes (Thermo Scientific).
The TFF process was scaled up to 1 L and 8 L using D02-P20U-05-N (140 cm2) and S02-P20U-05-N (1000 cm2) hollow fiber filters respectively (Repligen Corporation). Scale-up studies were performed using AAV9 material from the same 20 L cell culture harvest batch that was stored at -80 °C. The material was thawed and prepared using the lysis and clarification process listed above. The 1 L scale up run utilized size 16 tubing for the recirculation loop, permeate line and feed line. The crossflow rate was set to 225 mL/min to maintain 6000 s-1 shear rate and the permeate rate was started at 4.4 mL/min and slowly increased in 1 mL/min increments until a 50–60 LMH flux was achieved. The 8 L scale up run utilized size 18 tubing for the recirculation loop and size 16 tubing for the permeate and diafiltration lines. The crossflow rate was set to 1400 mL/min to maintain 6000 s-1 shear rate and the permeate rate was started at 4.4 mL/min and slowly increased in 1 mL/min increments until a 50–60 LMH flux was achieved.
Purification of AAV harvest material at Capsida Biotherapeutics
AAV cell culture harvest material was lysed and clarified using Capsida’s standard protocol and split for head-to-head comparison of IsoTag™AAV purification to affinity chromatography. One liter of clarified lysate was purified using the IsoTag™AAV TFF process described above and then buffer exchanged via TFF into a cesium chloride enrichment buffer. One liter of clarified lysate was prepared for affinity chromatography including a TFF concentration step prior to loading on an affinity chromatography column (Thermo resin). The AAV purified from both methods were run through the same platform downstream purification process including cesium chloride gradient ultracentrifugation and buffer exchange via dialysis. Process intermediate samples as well as final drug product from both purification streams were analyzed for yield using ddPCR and total capsid ELISA titers. Particle size and aggregation were analyzed using Unchained Lab’s Stunner. AAV purity and capsid integrity were analyzed via SDS-PAGE with Coomassie staining, CE-SDS, host cell protein ELISA, host cell DNA and qPCR.
Isolere Bio AAV analytical methods
Process efficiencies were determined by comparing the total amount of AAV in individual process fractions to the total amount of AAV in the starting harvest. IsoTag™ capture efficiency was determined by subtracting the amount of AAV in the flow-through from the total starting AAV and dividing the resulting captured AAV by the total AAV in the harvest. The elution yield was determined by dividing the amount of AAV in elution fractions by the total starting AAV amount in the harvest. Total capsid titer was measured using an AAV9 capsid ELISA kit (Progen, PRAAV9) per manufacturer’s protocol. Genome titer was determined via quantitative PCR assay using CFX Connect Real-Time PCR Detection System (Bio-Rad), AAV Universal ITR Primers (IDT) and SsoAdvanced SYBR Green Supermix (Bio-Rad).
Western blotting was also used to track AAV across process fractions. 10 uL samples from individual fractions were run in reducing conditions on 8% Bis-Tris Bolt 1.0 mm Mini Protein Gels (Invitrogen) and transferred onto PVDF using the Trans-Blot Turbo system (Bio-Rad) according to the manufacturer’s recommendation. Blots were probed with mouse monoclonal anti-AAV (VP1, VP2, VP3) B1 antibody (1:400, ARP) as the primary antibody and goat anti-mouse HRP (1:6666, Invitrogen) as the secondary antibody. Blots were developed using SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Scientific) and imaged using iBright imaging system (Thermo Scientific). AAV purity was determined using SDS-PAGE and host cell contaminant assays. SDS-PAGE was run on 8% Bis-Tris Bolt 1.0 mm Mini Protein Gels (Invitrogen) in 1X MOPS running buffer (Invitrogen) according to manufacturer’s recommendation. Gels were then stained with the SilverXpress silver staining kit (Thermo Scientific) according to the manufacturer’s protocol. Log reduction values (LRV) of host cell proteins (HCP) was determined using HEK 293 HCP ELISA kit (Cygnus). LRV of host cell DNA (HCD) was determined by assaying starting material and eluted material with Quant-iT PicoGreen dsDNA kit (Thermo Scientific).
Capsida Biotherapeutics AAV analytical methods
Product recoveries from each process step were determined by digital droplet PCR (ddPCR; Biorad) and capsid ELISA (custom in-house AAV capsid ELISA). Purity of the elution and final products were assayed using SDS- PAGE/Coomassie staining (NuPAGE 4–12% Bis- Tris, Invitrogen Novex SimplyBlue Safe Stain), capillary electrophoresis SDS (CE-SDS; PA800 Plus from AB Sciex), host-cell protein ELISA (Cygnus) and host-cell DNA quantitative PCR (qPCR-Thermo Scientific). Aggregation of the products was evaluated by dynamic light scattering using Unchained Lab’s Stunner instrument. Final product in vitro infectivity was tested by Tissue Culture Infection Dose 50% (TCID50) assay using HeLa RC32 cells and Taq- Man qPCR.
References
1. Gupta V, Lourenco SP, Hidalgo IJ. Development of Gene Therapy Vectors: Remaining Challenges. J. Pharm. Sci. 2021; 110(5), 1915–1920. Crossref
2. Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr. Gene Ther. 2013; 13(6), 453–468. Crossref
3. Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J. Transl. Med. 2016; 14(1), 288. Crossref
4. Zhou HS, Liu DP, Liang CC. Challenges and strategies: the immune responses in gene therapy. Med. Res. Rev. 2004; 24(6), 748–761. Crossref
5. Aucoin MG, Perrier M, Kamen AA. Improving AAV vector yield in insect cells by modulating the temperature after infection. Biotechnol. Bioeng. 2007,97(6), 1501–1509. Crossref
6. Merten OW. AAV vector production: state of the art developments and remaining challenges. Cell Gene Ther. Insights 2016; 2(5), 521–551. Crossref
7. Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998; 72(3), 2224–2232. Crossref
8. Singh N, Heldt CL. Challenges in downstream purification of gene therapy viral vectors. Curr. Opinion Chem. Eng. 2022;35. Crossref
9. Fuerstenau-Sharp M, Pettitt D, Bure K, Smith J, Ware J, Brindley D. Scalable purification of viral vectors for gene therapy: An appraisal of downstream processing approaches. BioProcess Int. 2017; 15(2). Crossref
10. Capra E, Gennari A, Loche A, Temps C. Viral-vector therapies at scale: Today’s challenges and future opportunities. McKinsey Insights 2022. Crossref
11. Ettre LS, Zlatkis A. 75 years of chromatography: a historical dialogue. 2011 Elsevier. Crossref
12. Kraemer EO, Lansing W. The Molecular Weight of Linear Macromolecules by Ultracentrifugal Analysis. I. Polymeric i-Hydroxydecanoic Acid1. J. Am. Chem Soc. 1933; 55(10), 4319–4326. Crossref
13. Stein R, Doty P. A Light Scattering Investigation of Cellulose Acetate1. J. Am. Chem. Soc. 1946; 68(2), 159–167. Crossref
14. Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999; 17(11), 1112–1115. Crossref
15. Bhattacharyya J, Bellucci JJ, Weitzhandler I et al. paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat. Commun. 2015,6, 7939. Crossref
16. Luginbuhl KM, Schaal JL, Umstead B et al. One-week glucose control via zero-order release kinetics from an injectable depot of glucagon-like peptide-1 fused to a thermosensitive biopolymer. Nat. Biomed. Eng. 2017; 1. Crossref
17. MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 2009; 8(12), 993–999. Crossref
18. Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010,62(15), 1479–1485. Crossref
19. Municoy S, Álvarez Echazú MI, Antezana PE et al. Stimuli-responsive materials for tissue engineering and drug delivery. International Journal of Molecular Sciences 2020; 21(13), 4724. Crossref
Affiliations
Jennifer Haley
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
JB Jones
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
Sophia Petraki
Capsida Biotherapeutics,
1300 Rancho Conejo Blvd.,
Thousand Oaks, CA 91320
Melissa Callander
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
Shaleen Shrestha
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
Emily Springfield
Capsida Biotherapeutics,
1300 Rancho Conejo Blvd.,
Thousand Oaks, CA 91320
Laura Adamson
Capsida Biotherapeutics,
1300 Rancho Conejo Blvd.,
Thousand Oaks, CA 91320
Ashutosh Chilkoti
Department of Biomedical
Engineering,
Duke University, Durham, NC 27708
Michael J Dzuricky
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
Kelli M Luginbuhl
Isolere Bio, Inc.,
4021 Stirrup Creek Dr,
Ste 210, Durham, NC 27703
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Haley J, Jones JB, Callander M, Shrestha S, Dzuricky MJ, and Luginbuhl KL were all employed by Isolere Bio, Inc. during their contributions to this work and they all own stock options in the company. Isolere Bio has received funding from Northpond Ventures and the North Carolina Biotech Center, as well as small business grants from NIH. Luginbuhl KM and Dzuricky MJ hold executive titles at Isolere Bio, Inc. Luginbuhl KM and Chilkoti A are founders of Isolere Bio and serve on the Board of Directors. Isolere Bio, Inc. has IP licensed from Duke University and Phase Bio Pharmaceuticals. Luginbuhl KM, Chilkoti A, and Dzuricky MJ are authors on patents licensed or owned by Isolere Bio, Inc. Only materials and information (no financial or other incentives) were exchanged by Capsida Therapeutics, Inc and Isolere Bio, Inc to carry out the comparison studies. The authors disclose no conflicts of interest.
Funding declaration: Research and development of the IsoTag™AAV process was in part funded by government grants from the NSF and NIH with grant numbers 1843074 and 1R44-GM139503-01A1 respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Isolere Bio, Inc. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Sep 7 2022; Revised manuscript received: Oct 18 2022; Publication date: Nov 3 2022.

