Augmenting Automated Analytics Using Fluorescent Nanosensors
Cell Gene Therapy Insights 2018; 4(9), 837-850.
10.18609/cgti.2018.085
Cell and gene therapies (CGTs) are projected to transform healthcare precision in the biotherapeutics sector. However, for their true potential to be realised, advancements must be made to optimising their manufacture, such that CGT production is precise, reproducible and robust. This includes monitoring and control of complex cell culture conditions, such as extracellular and subcellular biochemical parameters, for which there are no readily available automated analytical systems. Biosensors, such as fluorescent nanosensors, provide a tangible solution to augment CGT manufacture, as they enable off-line, online and inline monitoring of the cellular microenvironments. This expert insight highlights how the automated analytical afforded by fluorescent nanosensors, could permit real-time realignment of critical sub-cellular biochemical parameters to enhance CGT manufacture. The insight concludes by evaluating how the integration of fluorescent nanosensors with new and established methods could pave-the-way forward to maximise CGT potential.
Submitted for Peer Review :Sep 18 2018 Published: Nov 20 2018
INTRODUCTION
“To measure is to know … If you cannot measure it, you cannot improve it.” Lord William Thomson Kelvin, 1824–1907.
Measurement aims to quantify every aspect of our surroundings, from the distance to Andromeda, the Milky Way’s neighbouring galaxy, to the mass of a Z boson, a subatomic particle in the standard model of particle physics. Standardisation of the seven fundamental measures of length (meter), time (second), mass (kilogram) temperature (kelvin), electricity (ampere), light (candela) and amount (mole) has enabled the natural sciences and economies to find commonality in measurement units. Order can be established from the apparent disorder through the application of measurement tools to enhance the knowledge of the world we live in. Moreover, the iterative application of new and improved technologies to probe our surroundings has augmented our knowledge and our ability to influence it.
Biological systems, in particular the human body, have attracted the most attention with regard to the human race’s drive to understand and influence. Governments, pharmaceutical companies and academic institutions annually invest large sums of money into the development of new technologies and drugs with the aim to enhance longevity or eradicate diseases [1]. However, most of the changes that occur in a biological system, resulting in the development of healthy or diseased tissue, appear in microenvironments at the sub cellular level, which is at the current limit of our understanding. Bearing this in mind there are no readily available shortcuts to effectively manipulate biological systems in a controlled manner, without having some prior knowledge on how they operate. Therefore, to enhance our understanding of the building blocks of life, before strategies are implemented to influence them, sensors or techniques must be developed that are able to map the transport and micro-localisation of critical molecules and ions, which are essential for cellular function.
THERAPEUTIC DISCOVERY
Pharmaceutical companies have attempted to overcome this limit through employment of high throughput automated screening strategies that aim to examine vast numbers of natural and synthetic compounds with the hope they elicit a biological effect. However, this approach was not economically viable for sustained periods, as large amounts of money were invested with diminishing returns, yielding fewer successful drug candidates [2]. However, personalised medicine is changing the way the pharmaceutical industry aims to cure disease. Patients are stratified by characterizing their genetic or biochemical profile to identify the disease etiology. These measurements permit the development of targeted therapy to delivery functional proteins, cells and genes to repair, restore or facilitate the removal disease.
Proteins and viral vectors have been successfully produced using large-scale manufacture systems such as Pichia pastoris, Saccharomyces cerevisiae, Escherichia coli or mammalian cell culture. This is because, these systems are well defined and can be monitored effectively using extracellular parameters temperature, pH and dissolved oxygen concentrations, due to the excellent automated feedback. In contrast, cell therapies have been produced for a limited number of conditions. This is due to challenges in optimisation of complex cell culture conditions, which includes a multitude of intricate extracellular and sub-cellular biochemical parameters, for which there are no readily available automated monitoring systems, such that they have proved significantly more challenging to produce. Therefore, for their true potential to be realised advancements must be made optimizing subcellular sensory bioprocessing technology, which in turn will augment manufacturing capacity.
SENSORS
Sensors recognise stimuli in their surroundings, to transduce a signal to a detector, which can then be quantified and interpreted as a measurement. Ideally a sensor for biological measurement should:
- Provide high spatial and temporal resolution, so that dynamic measurements can be made from sub cellular microenvironments;
- Cause negligible cellular perturbations, such that measurements are independent of the sensing technique; and
- Demonstrate high sensitivity and selectivity to the analyte of interest, for accurate and precise quantitative measurements.
Paradigm shifts in the development of tools and techniques to manipulate and investigate matter at the micro- and nano-scale, such as scanning electron microscopy (SEM) [3], transmission electron microscopy (TEM) [4], scanning tunnelling microscopy (STM) [5], atomic force microscopy (AFM) [6], scanning ion conductance microscopy (SICM) [7] and optical microscopy [8], have permitted the development of miniaturised sensors, such as optical [9] and electrochemical sensors [10], which can be used to probe the micro-environments found in living cells. Examples of such a technology are optical nanosensors.
Optical nanosensors
Optical nanosensors are probes that have nanometre-scale sizes in all three dimensions [11] that utilise light in many forms, such as ultra-violet light, visible light, near-infrared and infra-red, to elucidate a vast amount of detail about the inner workings of microenvironments. Of the number of optical nanosensors reported in the literature, probes that utilise the principles of fluorescence and phosphorescence have shown the most promise [12–15]. This is largely due to:
- The high spatial and temporal resolutions they provide, when imaged with optical microscopes;
- Enhanced sensitivity, when compared with the weak signals obtained from other techniques such as absorption spectroscopy and Raman scattering; and
- Can be engineered to be non-invasive and highly specific to analytes of interest.
Due to the diversity of fluorescent sensing elements available, and ability of the versatile nanoparticle matrix to protect the sensing element, development of fluorescent nanosensors has been taken on by several research groups around the world and has permitted the development of ratiometric fluorescent nanosensors (Figure 1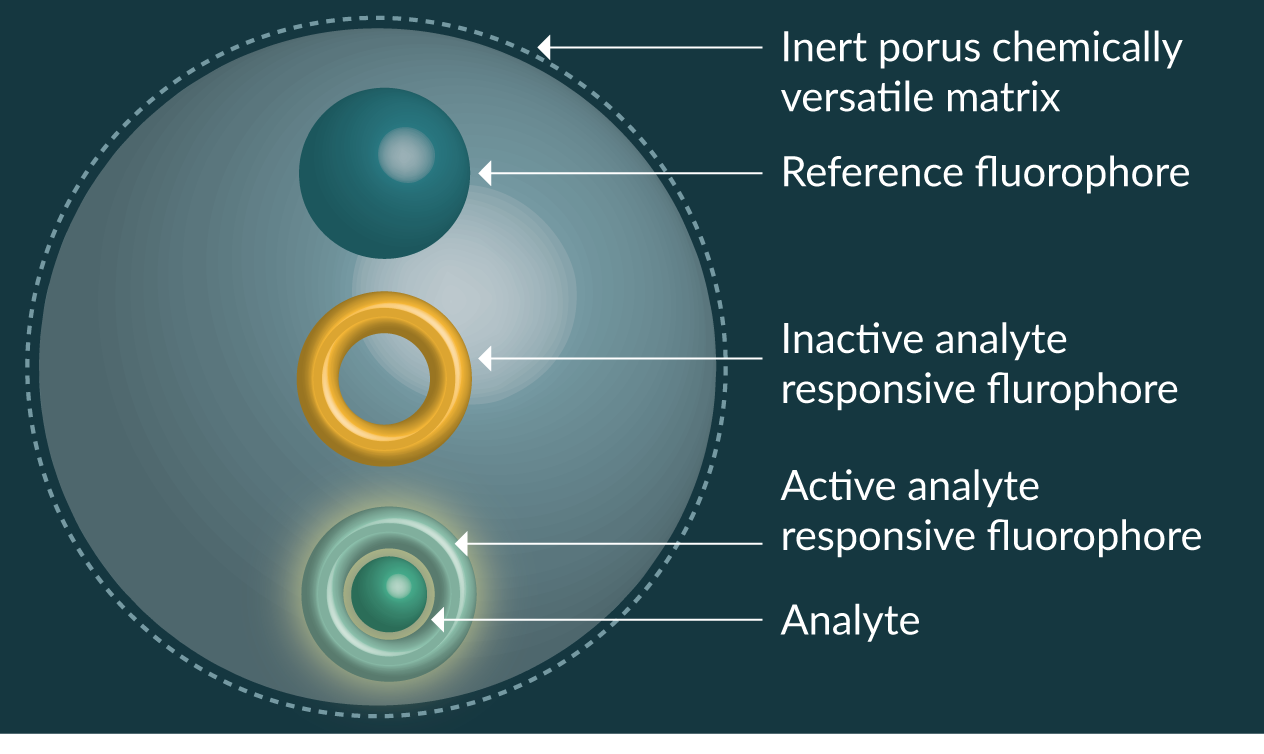
Fluorescent nanosensors have been reported for hydrogen ions (pH) [17,18], molecular oxygen [19], calcium [20,21], copper [22], chloride [23], glucose [24,25], iron [26–28], lead [29], magnesium [30], mercury [31], potassium [32], ROS [33–37], sodium [38,39], zinc [40], proteins [41,42], nucleic acids [43,44], ATP [45,46] and temperature [47–50]. The scope for producing new fluorescent nanosensors is limited only by the availability of analyte sensitive fluorophores or receptors that can transduce a signal to fluorescent molecules.
Delivery of nanosensors
Nanosensors have been known to spontaneously cross cell membranes through pinocytosis or phagocytosis. However, not all nanoparticles demonstrate this property. This is because the cellular uptake of nanoparticles is heavily influenced by size shape and charge [51,52]. Therefore a number of strategies have been explored to enhance the delivery in a controlled manner (Figure 2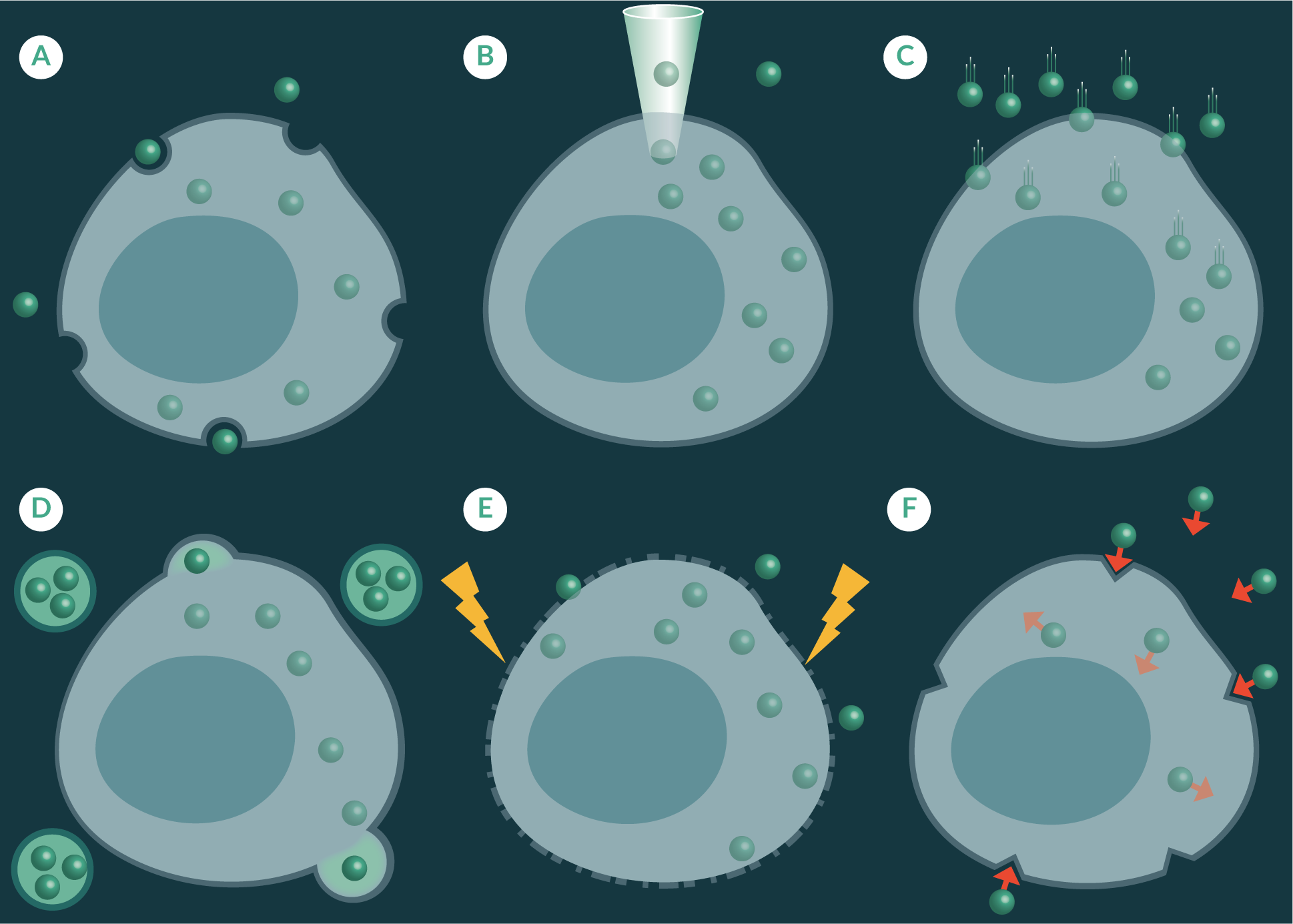
Pico-injection uses a fine needle to puncture the cell membrane to deliver pico-litres of sample into a single cell (Figure 2B) [53]. This method can cause unwanted cellular perturbations and require a high level of skill to inject a sample into a cell without causing excessive damage. Gene guns have predominantly been used to transfect cell cultures with deoxyribonucleic acid (DNA) [54] and plasmids [55], but their use has also been shown for the delivery of nanosensors across cell membranes [30]. The gene gun blasts dry nanoparticles into a cell culture dish using pressurized helium gas helium as a propellant (Figure 2C) [56]. This method has been found to maintain cell viability whilst delivering large quantities of nanoparticles across cell membranes [57]. It is important to note strategies such as pico-injection and gene delivery are low throughput and may be challenging to incorporate on large-scale autonomous cell production processes.
Liposomal transfection, like gene gun delivery, has also been used to deliver nucleic acids across cell membranes but have shown to be useful at transporting nanoparticles [53]. Liposomal transfection methods utilise artificially prepared vesicles formed from lipid bilayers, which envelop a small volume of nanosensors. The liposomal vesicles fuse with the cell membrane to transfer the contents of the liposome to the cellular cytoplasm (Figure 2D). Whereas, electroporation applies a voltage across a cell membrane to increase its permeability so that foreign material, such as fluorescent nanoparticles, can travel through to sub cellular spaces (Figure 2E), and has been shown to be effective at transporting foreign material across cell membranes of a number of cell lines, including yeast cells [45]. Another approach has been to utilize the versatile nanosensor matrix as a platform to attach chemical moieties that have demonstrated targeted uptake through receptor mediated uptake pathways (Figure 2F). Examples of some of these moieties which have been use to decorate nanoparticles are the universal membrane penetrating peptide trans-activating transcriptional activator (TAT), derived from Human immunodeficiency virus 1 (HIV-1) [58], and the tumour-homing F3-peptide, which is derived from high mobility group nucleosome binding protein 2 (HMGN2) [59].
Due to the number of mechanisms available for nanoparticle delivery, nanosensors have been applied to a range of biological systems including mammalian cell lines, [33,60] stem cells [61], scaffolds for regenerative medicine [62–64] and model organisms [65,66]. It is important to note targeted placement of probes adjacent to subcellular organelles of interest, such as mitochondria, is an even more challenging. Therefore, subcellular positioning must also be considered if specific bioprocesses are to be monitored [67]. Effective monitoring of critical molecules and ions in situ or in vitro in model biological systems, such as the ones mentioned above, using fluorescence microscopy could generate diagnostic information biological function and suggest new approaches to enhance cell and gene therapy culture.
INTERGRATING NANOSENSORS FOR CELL & GENE THERAPY
Optimized cell culture encompasses the production of sufficient quantities of viable cells, which have the desired function or therapeutic activity, whilst effectively utilising resources, such as raw materials and time [68]. Therefore, integration of sensors to automatically feedback will enhance cell culture consistency and capacity and concurrently reduce waste of essential resources.
Bioreactors & fluorescent nanosensors
Cell production is typically conducted using bioreactors [69], which are vessels that permit cell expansion by providing the essential culture conditions (e.g., temperature, aerobic/aerobic & pH), nutrients (e.g., carbohydrates, proteins and lipids) and growth factors (e.g., cytokines) [70]. Monitoring of cell culture environments in bioreactors can occur via direct or indirect measurements, as well as offline, online and inline observations [71].
Bioreactors are selected or designed based on the cell culture conditions and required scale of manufacture. Cell culture conditions refers to the ex vivo environments cells prefer to enhance their expansion. For non-adherent cells to homogenise culture conditions this can include the introduction of sheer stress, which can also improve mass distribution of nutrients and growth factors through the culture medium (e.g., orbital shakers, spinner flask and rotating wall vessels) [72]. Whereas rocking [73] or perfusing [74] culture media has also proved effective for homogenization of adherent cells. Hybrid bioreactors that increase the surface area for cell attachment through the introduction of microcarriers [75] and scaffolds [76] that are distributed throughout high sheer environments have also been introduced to improve culture capacity and maximise nutrient and growth factor resource allocation. Scale of manufacture is dependent on ultimate use of cells. Small-scale bioreactors are used for in vitro research purposes or clinical allogeneic products in commercial settings, such as chimeric antigen receptor (CAR) T cells [77]. Whereas, large-scale manufacture, which can reach batch sizes of 20,000 liters, are typically used for commercial projects [78].
Direct & indirect measurement
Direct measurement corresponds to whether information on culture parameters is determined on actual measurement of key components (e.g., the partial pressure of oxygen or carbon dioxide) in the bioreactor or cells [79]. Whereas indirect measurement corresponds to evaluation of changes in cell cultures marker (e.g., cell surface expression marker and excreted products) after the input parameters have been modified. Therefore, direct measurements permit measurement of real-time events, which can then then be used for automated optimization of cultures. Whereas indirect measurements can provide information of cell behaviour due to changing growth parameters; however, these may include measurement artefacts as a number of intricate changes can affect the ultimate cell output, unless defined biochemical pathways have been identified [80]. Indirect measurements can also be used for real-time optimization of cell cultures, however, as biochemical processes contain an inherent time lag, immediate optimization of growth parameters may be challenging [80].
When fluorescent nanosensors are delivered to subcellular spaces, they permit direct and indirect quantification of biochemical parameters that could be used to for real-time optimization of growth parameters. Direct measurement of biochemical processes is evidenced by measurement of pH in Saccharomyces cerevisiae [81], whereas indirect measurement of has been shown by the quantification of hydrogen peroxide in human mesenchymal stem cells [82], a toxic by-product of porphyrin induced photodynamic light therapy.
Offline, online & inline analytics
Offline cell culture is a form of indirect measurement, where samples are extracted from the bioreactor and analysed at an independent location [83]. Extraction of sample can permit a greater variety of analytics to be conducted, as samples can be subjected to an array of assays that cannot be readily integrated into the bioreactor. However, when the extraction vessel is different from the bioreactor, especially if the growth conditions are altered and the samples are not stable, interaction with new environments may introduce measurement artefacts. Consequentially, this may hinder utility of newly acquired analytics for optimization growth parameters. Furthermore, extraction away from the bioreactor reduces the potential for real-time feedback and reduces sample size as it cannot be return to the culture vessel.
The automated analytics provided by direct online monitoring permits real-time in situ measurement of analytes of interests. This closes the feedback loop, or permits automated analytics, such that growth conditions and parameters can be attenuated to optimise cell growth conditions [84]. Inline monitoring, a direct measurement technique, can be described as a hybrid offline/online measurement systems, where cell culture systems are continually fed and monitored at a location adjacent to or within the bioreactor and can be recirculated into the growth vessel after analysis, preventing loss of sample [85]. Inline systems are useful when bulk cell culture system are turbid, preventing spectroscopic analysis, or possess high fluid flow rates during mixing, which might generate unwanted measurement artefacts. Cell culture parameters are automatically analysed and attenuated to optimise growth. Due to the automated analytics afforded by online and inline monitoring, both cell growth and resource management are optimized.
Implementation of nanosensors for cell therapy
Fluorescent nanosensors can be incorporated in sub cellular spaces or distributed throughout the system to provide feedback of extracellular parameters [86]. Delivery of fluorescent nanosensors to subcellular spaces is a resource efficient method of obtaining cell specific information, especially for research purposes. This is because, although fluorescent nanosensors have demonstrated low toxicity [87], their ultimate use in humans is yet to be determined. Therefore, for mass production of cells for clinical use an alternative measurement system may be required. However, when fluorescent nanosensors are distributed throughout the bioreactor, vast quantities of particles will be required. These particles could be recovered for repeat use, but in reality, this may not be possible as they may introduce biologically active contaminants, which may subsequently alter the viability of batches.
Fluorescent nanosensors could be used for offline, online and inline measurement for cell culture parameters in bioreactors (Figure 3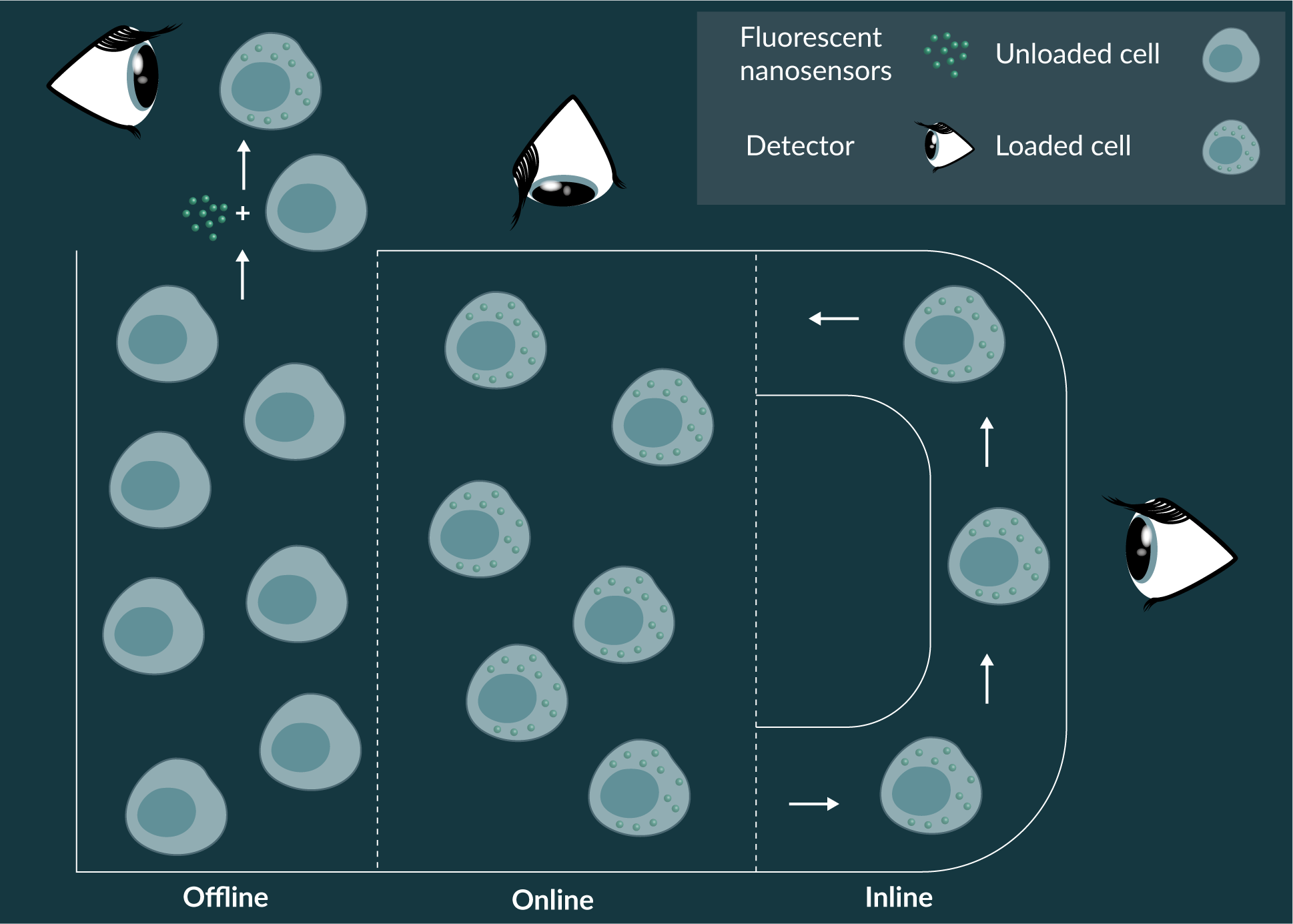
Fluorescent nanosensors are ideally suited to online and inline measurement systems for research purposes and prior to large-scale clinical application. Cell measurements could be conducted by direct online measurement of culture system with fluorescent microscopy or spectroscopy or inline using methods such as integrated fluorescent flow cytometry. Bioreactors that permit visualisation of growth conditions have been developed [90], whereas bioreactors with integrated flow cytometers are emerging for research purposes. From a practical point of view, offline fluorescent nanosensor technologies would need to address an analytical niche currently occupied by powerful established methods, such as flow cytometry, liquid chromatography, and spent media analysis. Therefore, fluorescent nanosensors could be utilized for established techniques, such as flow cytometry, when conventional antibody-based tools are not available or are not able to perform the dynamic biochemical parameter measurements afforded by fluorescent nanosensors.
FUTURE PERSPECTIVE
Biosensors such as fluorescent nanosensors are the future of cell and gene therapy manufacture as they enable online and inline monitoring of the cellular microenvironment. Automated analytics of culture environments permits real-time realignment of ideal biochemical parameters, through data driven allocation of key resources, such as nutrients and growth factors, as well as eliminating waste. It is anticipated the developments in this field provide great promise to further understand the cellular microenvironment and will pave the way forward for cell and gene therapies.
“Science has given to him an acquaintance with the different relations of the parts of the external world; and more than that, it has bestowed upon him powers which may be almost called creative; which have enabled him to modify and change the beings surrounding him, and by his experiments to interrogate nature with power, not simply as a scholar, passive and seeking only to understand her operations, but rather as a master, active with his own instruments,” Sir Humphry Davy (1778–1829) [91]:
ACKNOWLEDGEMENTS
Funding from the UK Engineering and Physical Sciences Research Council (EPSRC) for the Future Targeted Healthcare Manufacturing Hub is gratefully acknowledged (Grant Reference No. EP/P006485/1), (VMC). Financial and in-kind support from the consortium of industrial users is also acknowledged by Veeren M Chauhan. He also gratefully acknowledges Dr Jonathan W Aylott for his continued financial support and academic guidance.
financial & competing interests disclosure
The author has no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003; 22(2): 151–85. CrossRef
2. Bleicher KH, Bohm HJ, Muller K, Alanine AI. Hit and lead generation: Beyond high-throughput screening. Nat. Rev. Drug Discov. 2003; 2(5): 369–78. CrossRef
3. Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. Plos. Biol. 2004; 2(11): 1900–9. CrossRef
4. Wang ZL. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2000; 104(6): 1153–75. CrossRef
5. Ito E, Takahashi T, Hama K, Yoshioka T, Mizutani W, Shimizu H, Ono M. An approach to imaging of living cell-surface topography by scanning tunneling microscopy. Biochem. Biophy. Res. Commun. 1991; 177(2): 636–43. CrossRef
6. A-Hassan E, Heinz WF, Antonik MD et al. Relative microelastic mapping of living cells by atomic force microscopy. Biophys. J. 1998; 74(3): 1564–78. CrossRef
7. Korchev YE, Bashford CL, Milovanovic M, Vodyanoy I, Lab MJ. Scanning ion conductance microscopy of living cells. Biophys. J. 1997; 73(2): 653–8. CrossRef
8. Huang B, Bates M, Zhuang XW. Super-resolution fluorescence microscopy. Ann. Rev. Biochem. 2009; 78: 993–1016. CrossRef
9. Cullum BM, Vo-Dinh T. The development of optical nanosensors for biological measurements. Trends Biotechnol. 2000; 18(9): 388–93. CrossRef
10. Rodriguez-Mozaz S, Marco MP, de Alda MJL, Barcelo D. Biosensors for environmental applications: Future development trends. Pure Appl. Chem. 2004; 76(4): 723–52. CrossRef
11. Cullum BM. Dekker Encyclopedia of Nanoscience and Nanotechnology, 2nd Edition ed., CRC Press, Boca Raton, UK, 2009. CrossRef
12. Aylott JW. Optical nanosensors – an enabling technology for intracellular measurements. Analyst 2003; 128(4): 309–12. CrossRef
13. Lee YEK, Smith R, Kopelman R. Nanoparticle PEBBLE Sensors in live cells and in vivo. Ann. Rev. Anal. Chem. 2009; 2: 57–76. CrossRef
14. Korzeniowska B, Schulz A, Wencel D, McDonagh C. Ieee, Luminescent nanoparticle-based intracellular sensing. Ieee Sensors 2011; 1709–11. CrossRef
15. Schulz A, Wotschadlo J, Heinze T, Mohr GJ. Fluorescent nanoparticles for ratiometric pH-monitoring in the neutral range. J. Mater. Chem. 2010; 20(8): 1475–82. CrossRef
16. Chauhan VM, Giuntini F, Aylott JW. Quadruple labelled dual oxygen and pH-sensitive ratiometric nanosensors. Sensing Bio. Res. 2016; 8: 36–42. CrossRef
17. Clark HA, Kopelman R, Tjalkens R, Philbert MA. Optical nanosensors for chemical analysis inside single living cells. 2. Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal. Chem. 1999; 71(21): 4837–43. CrossRef
18. Elsutohy MM, Selo A, Chauhan VM et al. Enhanced distance-dependent fluorescence quenching using size tuneable core shell silica nanoparticles. RSC Advances 2018; 8(62) 35840–35848. CrossRef
19. Xu H, Aylott JW, Kopelman R, Miller TJ, Philbert MA. A real-time ratiometric method for the determination of molecular oxygen inside living cells using sol-gel-based spherical optical nanosensors with applications to rat C6 glioma. Anal. Chem. 2001; 73(17): 4124–33. CrossRef
20. Webster A, Compton SJ, Aylott JW. Optical calcium sensors: development of a generic method for their introduction to the cell using conjugated cell penetrating peptides. Analyst 2005; 130(2): 163–70. CrossRef
21. Schulz A, Woolley R, Tabarin T, McDonagh C. Dextran-coated silica nanoparticles for calcium-sensing. Analyst 2011; 136(8): 1722–7. CrossRef
22. Seo S, Lee HY, Park M et al. Fluorescein-Functionalized Silica Nanoparticles as a Selective Fluorogenic Chemosensor for Cu2+ in Living Cells. Eur. J. Inorgan. Chem. 2010; (6): 843–7. CrossRef
23. Graefe A, Stanca SE, Nietzsche S et al. Development and critical evaluation of fluorescent chloride nanosensors. Anal. Chem. 2008; 80(17): 6526–31. CrossRef
24. Xu H, Aylott JW, Kopelman R. Fluorescent nano-PEBBLE sensors designed for intracellular glucose imaging. Analyst 127(11) (2002) 1471-1477. CrossRef
25. Brown JQ, Srivastava R, McShane MJ. Encapsulation of glucose oxidase and an oxygen-quenched fluorophore in polyelectrolyte-coated calcium alginate microspheres as optical glucose sensor systems. Biosens. Bioelectronics 2005; 21(1): 212–6. CrossRef
26. Sumner JP, Kopelman R. Alexa Fluor 488 as an iron sensing molecule and its application in PEBBLE nanosensors. Analyst 130(4) (2005) 528-533. CrossRef
27. Shamsipur M, Molaei K, Molaabasi F et al. preparation and characterization of new green emitting carbon dots for sensitive and selective off/on detection of Fe3+ ion and ascorbic acid in water and urine samples and intracellular imaging in living cells. Talanta 2018; 183: 122–30. CrossRef
28. Xia J, Zhuang YT, Yu YL, Wang JH. Highly fluorescent carbon polymer dots prepared at room temperature, and their application as a fluorescent probe for determination and intracellular imaging of ferric ion. Microchimica Acta 2017; 184(4): 1109–16. CrossRef
29. Brenneman KL, Poduri S, Stroscio MA, Dutta, M. Optical Detection of Lead(II) Ions Using DNA-Based Nanosensor. Ieee Sensors J. 2013; 13(5): 1783–6. CrossRef
30. Park EJ, Brasuel M, Behrend C, Philbert MA, Kopelman R. Ratiometric optical PEBBLE nanosensors for real-time magnesium ion concentrations inside viable cells. Anal. Chem. 2003; 75(15): 3784–91. CrossRef
31. Li M, Wang Q, Shi X, Hornak LA, Wu N. Detection of Mercury(II) by Quantum Dot/DNA/Gold Nanoparticle Ensemble Based Nanosensor Via Nanometal Surface Energy Transfer. Anal. Chem. 2011; 83(18): 7061–5. CrossRef
32. Brown JQ, McShane MJ. Core-referenced ratiometric fluorescent potassium ion sensors using self-assembled ultrathin films on europium nanoparticles. Ieee Sensors J. 2005; 5(6): 1197–205. CrossRef
33. Josefsen LB, Aylott JW, Beeby A et al. Porphyrin-nanosensor conjugates. New tools for the measurement of intracellular response to reactive oxygen species. Photochemical & Photobiological Sciences 2010; 9(6): 801–11. CrossRef
34. Liu HM, Wang BC, Li DH et al. MoS2 nanosheets with peroxidase mimicking activity as viable dual-mode optical probes for determination and imaging of intracellular hydrogen peroxide. Microchimica Acta 2018; 185(6): 9. CrossRef
35. Wang XH, Peng HS, Yang W, Ren ZD, Liu YA. Mitochondria-targeted theranostic nanoparticles for optical sensing of oxygen, photodynamic cancer therapy, and assessment of therapeutic efficacy. Microchimica Acta 2016; 183(10): 2723–31. CrossRef
36. Ping JT, Peng HS, Qin JL et al. A fluorescent nanoprobe for real-time monitoring of intracellular singlet oxygen during photodynamic therapy. Microchimica Acta 2018; 185(5): 8. CrossRef
37. Chen HY, Wu SH, Chen CT. Horseradish Peroxidase-Encapsulated Hollow Silica Nanospheres for Intracellular Sensing of Reactive Oxygen Species. Nanoscale Res. Lett. 2018; 13: 10. CrossRef
38. Buck SM, Koo YEL, Park E et al. Optochemical nanosensor PEBBLEs: photonic explorers for bioanalysis with biologically localized embedding. Curr. Opin. Chem. Biol. 2004; 8(5): 540–6. CrossRef
39. Lamy CM, Sallin O, Loussert C, Chatton J-Y. Sodium Sensing in Neurons with a Dendrimer-Based Nanoprobe. Acs Nano 2012; 6(2): 1176–87. CrossRef
40. Sumner JP, Aylott JW, Monson E, Kopelman R. A fluorescent PEBBLE nanosensor for intracellular free zinc. Analyst 2002; 127(1): 11–6. CrossRef
41. Welser K, Adsley R, Moore BM, Chan WC, Aylott JW. Protease sensing with nanoparticle based platforms. Analyst 2011; 136(1): 29–41. CrossRef
42. Welser K, Perera MDA, Aylott JW, Chan WC. A facile method to clickable sensing polymeric nanoparticles. Chem. Commun. 2009; (43): 6601–3. CrossRef
43. Wang RE, Zhang Y, Cai J, Cai W, Gao T. Aptamer-Based Fluorescent Biosensors. Curr. Med. Chem. 2011; 18(27): 4175–84. CrossRef
44. Liu JT, Du P, Zhang J, Shen H, Lei JP. Sensitive detection of intracellular microRNA based on a flowerlike vector with catalytic hairpin assembly. Chem. Commun. 2018; 54(20): 2550-–3. CrossRef
45. Nielsen LJ, Olsen LF, Ozalp VC. Aptamers Embedded in Polyacrylamide Nanoparticles: A Tool for in Vivo Metabolite Sensing. Acs Nano 2010; 4(8): 4361–70. CrossRef
46. V.C. Ozalp, L.J. Nielsen, L.F. Olsen, An Aptamer-Based Nanobiosensor for Real-Time Measurements of ATP Dynamics. Chembiochem 2010; 11(18): 2538–41. CrossRef
47. X. Wang, O. Wolfbeis, R. Meier, Luminescent probes and sensors for temperature. Chemical Society Reviews (2013). CrossRef
48. Fischer LH, Harms GS, Wolfbeis OS. Upconverting nanoparticles for nanoscale thermometry. Angewandte Chemie-International Edition 2011; 50(20): 4546–51. CrossRef
49. Oyama K, Takabayashi M, Takei Y et al. Walking nanothermometers: spatiotemporal temperature measurement of transported acidic organelles in single living cells. Lab Chip 2012; 12(9): 1591–3. CrossRef
50. Uchiyama S, Gota C. Luminescent molecular thermometers for the ratiometric sensing of intracellular temperature. Rev. Anal. Chem. 2017; 36(1): 18. CrossRef
51. Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters 2006; 6(4): 662–8. CrossRef
52. Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules 2008; 9(2): 435–43. CrossRef
53. Webster A, Coupland P, Houghton FD, Leese HJ, Aylott JW, The delivery of PEBBLE nanosensors to measure the intracellular environment, Cojnference on Bionanotechnology – From Self-Assembly to Cell Biology. Portland Press Ltd, Cambridge, ENGLAND, 2007; 538–43. CrossRef
54. O’Brien J, Lummis SCR. Biolistic and diolistic transfection: using the gene gun to deliver DNA and lipophilic dyes into mammalian cells. Methods 2004; 33(2): 121–5. CrossRef
55. Wirth MJ, Wahle P. Biolistic transfection of organotypic cultures of rat visual cortex using a handheld device. J. Neurosci. Meth. 2003; 125(1-2): 45–54. CrossRef
56. Klein RM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic acids into living cells. 1987, Biotechnology (Reading, Mass.) 1992; 24: 384–6. CrossRef
57. Clark HA, Hoyer M, Parus S, Philbert MA, Kopelman M. Optochemical nanosensors and subcellular applications in living cells. Mikrochimica Acta 1999; 131(1-2): 121–8. CrossRef
58. Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988; 55(6): 1189–93. CrossRef
59. Porkka K, Laakkonen P, Hoffman JA, BernasconiM, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Prod. Natl Acad. Sci. USA 2002; 99(11): 7444–9. CrossRef
60. Hou BH, Takanaga H, Grossmann G. Optical sensors for monitoring dynamic changes of intracellular metabolite levels in mammalian cells. Nature Protocols 2011; 6(11): 1818–33. CrossRef
61. Coupland PG, Fisher KA, Jones DRE, Aylott JW. Internalisation of polymeric nanosensors in mesenchymal stem cells: Analysis by flow cytometry and confocal microscopy. J. Controlled Rel. 2008; 130(2): 115–20. CrossRef
62. Harrington HC, Cato PA, Aylott JW, Rose FRAJ, Ghaemmaghami AM. Development of an immune responsive 3D human lung model. J. Tiss. Eng. Regen. Med. 2012; 6. CrossRef
63. Harrington HC, Rose FRAJ, Ghaemmaghami AM, Aylott JW. Monitoring analyte concentration using self reporting scaffolds a 3D culture model. J. Tiss. Eng. Regen. Med. 2012; 6: 366. CrossRef
64. Harrington HC, Rose FRAJ, Reinwald Y, Buttery LDK, Ghaemmaghami AM, Aylott JW. Electrospun PLGA fibre sheets incorporating fluorescent nanosensors: self-reporting scaffolds for application in tissue engineering. Anal. Methods 2013; 5(1): 68–71. CrossRef
65. Chauhan VM, Orsi G, Brown A, Aylott JW. Mapping the Pharyngeal and Intestinal pH of Caenorhabditis elegans and Real-Time Luminal pH Oscillations Using Extended Dynamic Range pH-Sensitive Nanosensors. ACS Nano 2013; 7(6): 5577–87. CrossRef
66. Lavado AS, Chauhan VM, Zen AA et al. Controlled intracellular generation of reactive oxygen species in human mesenchymal stem cells using porphyrin conjugated nanoparticles. Nanoscale 7(34) (2015) 14525–31. CrossRef
67. Wang XH, Peng HS, Yang L et al. Targetable Phosphorescent Oxygen Nanosensors for the Assessment of Tumor Mitochondrial Dysfunction By Monitoring the Respiratory Activity. Angewandte Chemie-International Edition 2014; 53(46): 12471–5. CrossRef
68. Gallo-Ramirez LE, Nikolay A, Genzel Y, Reichl U. Bioreactor concepts for cell culture-based viral vaccine production. Expert Rev. Vaccines 2015; 14(9): 1181–95. CrossRef
69. Ahmed S, Chauhan VM, Ghaemmaghami AM, Aylott JW. New generation of bioreactors that advance extracellular matrix modelling and tissue engineering Biotechnol. Lett. 2018; 1–25. CrossRef
70. Salehi-Nik N, Amoabediny G, Pouran B et al. Engineering Parameters in Bioreactor’s Design: A Critical Aspect in Tissue Engineering. Biomed. Res. Int. 2013; 15. CrossRef
71. Junker BH, Wang HY. Bioprocess monitoring and computer control: Key roots of the current PAT initiative. Biotechnol. Bioeng. 2006; 95(2): 226–61. CrossRef
72. Sucosky P, Osorio DF, Brown JB, Neitzel GP. Fluid mechanics of a spinner-flask bioreactor. Biotechnol. Bioeng. 2004; 85(1): 34–46. CrossRef
73. Oosterhuis NMG. Perfusion Process Design in a 2D Rocking Single-Use Bioreactor. In: G. Subramanian (Ed.). Continuous Processing in Pharmaceutical Manufacturing, Wiley-V C H Verlag Gmbh, Weinheim, 2015; 155–63. CrossRef
74. Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003; 9(3): 549–54. CrossRef
75. Schop D, Janssen FW, Borgart E, de Bruijn JD, van Dijkhuizen-Radersma R. Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J. Tiss. Eng. Regen. Med. 2008; 2(2-3): 126–35. CrossRef
76. Lin CH, Hsu SH, Huang CE, Cheng WT, Su JM. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials 2009; 30(25): 4117–26. CrossRef
77. Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018; 2(6): 377–91. CrossRef
78. Harrison RP, Chauhan VM. Enhancing cell and gene therapy manufacture through the application of advanced fluorescent optical sensors. Biointerphases 2018; 13(1): 8. CrossRef
79. Shaegh SAM, De Ferrari F, Zhang YS et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016; 10(4): 14. CrossRef
80. Rowland-Jones RC, van den Berg F, Racher AJ, Martin EB, Jaques C. Comparison of spectroscopy technologies for improved monitoring of cell culture processes in miniature bioreactors. Biotechnol. Prog. 2017; 33(2): 337–46. CrossRef
81. Elsutohy MM, Chauhan VM, Markus R et al. Real-time measurement of the intracellular pH of yeast cells during glucose metabolism using ratiometric fluorescent nanosensors. Nanoscale 2017; 9(18): 5904–11. CrossRef
82. Lavado AS, Chauhan, VM, Zen AA et al. Controlled Intracellular Generation of Reactive Oxygen Species in Human Mesenchymal Stem Cells Using Porphyrin Conjugated Nanoparticles. Nanoscale (2015). CrossRef
83. Kara S, Mueller JJ, Liese A. Online analysis methods for monitoring of bioprocesses. Chimica Oggi-Chemistry Today 2011; 29(2): 38–41. CrossRef
84. Heathman TFJ, Rafiq QA, Chan AKC et al. Characterization of human mesenchymal stem cells from multiple donors and the implications for large scale bioprocess development. Biochem. Eng. J. 2016; 108: 14–23. CrossRef
85. Lourenco ND, Lopes JA, Almeida CF, Sarraguca MC, Pinheiro HM. Bioreactor monitoring with spectroscopy and chemometrics: a review. Anal. Bioanal. Chem. 2012; 404(4): 1211–37. CrossRef
86. Clark HA, Barker SLR, Brasuel M et al. Subcellular optochemical nanobiosensors: probes encapsulated by biologically localised embedding (PEBBLEs). Sensors Act. B-Chem. 1998; 51(1–3): 12–6. CrossRef
87. Benjaminsen RV, Sun H, Henriksen JR, Christensen NM, Almdal K, Andresen TL. Evaluating Nanoparticle Sensor Design for Intracellular pH Measurements. Acs Nano 2011; 5(7): 5864–73. CrossRef
88. Meyer D, Hagemann A, Kruss S. Kinetic Requirements for Spatiotemporal Chemical Imaging with Fluorescent Nanosensors. Acs Nano 2017; 11(4): 4017–27. CrossRef
89. Desai AS, Chauhan VM, Johnston APR, Esler T, Aylott JW. Fluorescent nanosensors for intracellular measurements: synthesis, characterization, calibration, and measurement. Front. Physiol. 2013; 4: 401. CrossRef
90. Sbrana T, Ahluwalia A. Methods for Conducting Connected Culture Experiments Using the Quasi-Vivo (R) Chambers. In: Haycock JW, Ahluwalia A, Wilkinson JM (Eds.), Cellular in Vitro Testing: Methods and Protocols, Crc Press-Taylor & Francis Group, Boca Raton, 2013; 1–14. CrossRef
91. Davy H, Discourse A. Introductory to a Course of Lectures on Chemistry: Delivered in the Theatre of the Royal Institution on the 21st of January 1802, House of the Royal Institution 1802. CrossRef
Affiliation
Veeren M Chauhan
School of Pharmacy, University of Nottingham, Boots Science Building, Science Road, NG7 2RD, UK E-mail: veeren.chauhan@nottingham.ac.uk