Ultra-rapid microbial detection in cell & gene therapy products: the closest you can be to real-time release
Cell and Gene Therapy Insights 2023; 9(2), 207;
DOI: 10.18609/cgti.2023.31
Published: 26 March 2023
FastFacts
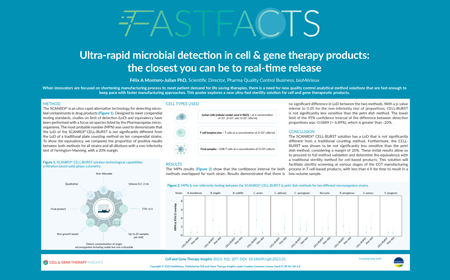 | Watch the video or read the poster to learn about:
|
