Translating Regenerative Medicine Science into Clinical Practice: the Local to Global pivot
Cell Gene Therapy Insights 2018; 4(5), 469-483.
10.18609/cgti.2018.043
Research into cell and gene therapies is globally dispersed, which creates scientific opportunities, but in turn, significant commercial challenges. In order to successfully bring promising scientific endeavors through to commercial opportunity, greater cross-border coordination of supply side activity considerations such as academic institutions, funding gaps, intellectual property, and commercial entities as well as demand-side issues of reimbursement, regulatory policy, stakeholder engagement and patient engagement should be advocated for.
Submitted for peer review: April 12 2018 Published: July 3 2018
After decades of scientific advance but commercial mis-starts, the field of regenerative medicine is finally offering more than simply a promise of transformative healthcare [1,2]. Recent commercial advances in CAR T-cell therapy based on chimeric antigen receptors for cancer treatment, such as the FDA’s 2017 approval of Novartis’s Kymriah (tisagenlecleucel; licensed from the University of Pennsylvania) [3], may now be the tip of the iceberg in emerging opportunities to treat conditions that have few traditional clinical options. Multiple treatments based on technologies such as cell therapy, gene therapy, and tissue engineered products finally appear to be emerging from the lab into the healthcare market. Yet, if we do not learn from the failures in the past, the recent advances could stall again [4].
A succinct timeline of cell therapy, gene therapy and tissue engineering is shown in Figure 1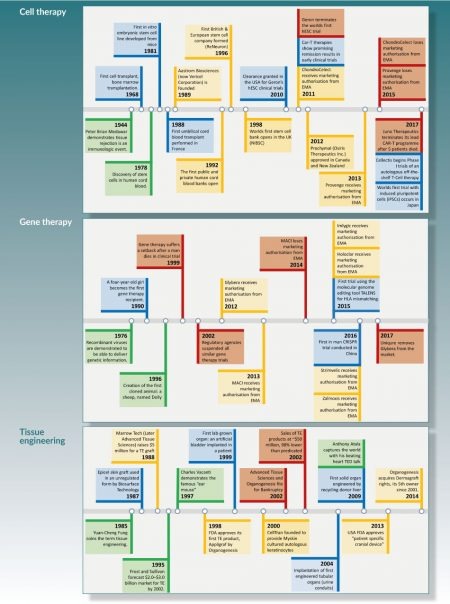
The global dispersion of regenerative medicine research is a strength in generating scientific breakthroughs, while also raising commercial challenges. Worldwide, three hubs stand out for bio-sciences: Boston-Cambridge in Massachusetts, the San Francisco Bay Area in California, and the ‘Golden Triangle’ defined by London, Cambridge, and Oxford in the UK [5,6]. The scientific strength in these hubs have bred ecosystems of venture funding and specialized human capital that support commercial translation from the lab to the market. In regenerative medicine, the Boston-Cambridge region is the undoubted leader able to attract globally mobile research, clinical expertise, industry, and funding. San Francisco has the benefit of access to the diverse range of technology start-ups in the Silicon Valley. For Europe, the ‘Golden Triangle’ stands out as the cornerstone of the industry on the continent.
Yet, key elements of regenerative medicine science span the globe far beyond these centres, including bases in other parts of the U.S., as well as in Japan, Sweden, Australia, Canada, Israel, Germany, and many other countries. Successful commercialization of products based on regenerative medicine science typically requires integration of advances from multiple geographies. This trend is clearly evidenced by the accelerating pace of mergers and acquisitions within the field. Acquisitions include not only product and technology portfolios such as Universal Cells by Astellas Pharma [7] and Kite Pharma by Gilead [8], but also the acquisition of manufacturing assets such as Progenitor Cell Technologies by Hitachi Chemical Energy Technology Co. [9]. Nonetheless, although there have been some successes, the commercial support ecosystem has often struggled to integrate across the multiple regions.
To foster innovation in the regenerative medicine sector, we advocate an emphasis on strategies that embrace an increasingly globally coordinated perspective. These strategies need to include supply side considerations of academic institutions, funding gaps, intellectual property, and commercial vendors as well as demand side issues of reimbursement, regulatory policy, stakeholder engagement, and patient engagement. This article discusses current weaknesses, recent advances, and potential future pathways. By detailing these areas, we advocate an increasingly strategic and collaborative approach to innovation across the global regenerative medicine sector.
SUPPLY SIDE CHALLENGES: GETTING TO THE MARKET
Academic institutions
Universities and medical schools throughout the world are major centres for regenerative medicine research that has potential for translation into clinical practice [10]. Yet academic institutions commonly struggle to commercialise research effectively. Indeed, most University Technology Transfer Offices run at a loss [11].
In part, the marketplace challenges occur because much of the research that university-based scientists undertake in is not commercially viable, at least in the near term. Instead, academic research commonly provides a base of science that may contribute to applications only in the distant future or, indeed, not at all [12].
A more focal problem, though, lies in integrating regenerative medicine projects with near term commercial potential that span multiple institutions, often in multiple countries. The issue is that academic commercialization efforts typically focus on individual institutions. The key challenge here lies in developing protocols and partnerships for combining intellectual property that spans institutional boundaries.
Fortunately, some trans-institutional models are emerging. A salient example is the German Fraunhofer Society which utilises the ‘Fraunhofer Model’ [13] to link more than 60 semi-autonomous institutes with universities and industries. The power of this model comes from the enhanced ability to exploit knowledge based opportunities through linking otherwise fragmented organisations [14]. This trend towards collaborative enterprise in academic institutions shows signs of expanding, with initiatives such as the emerging European Research Area encouraging combined research proposals across the European continent [15].
Funding gaps
Part of the inherent challenge with the commercialisation pathway is financial. Regenerative medicine diverges from some sectors in that new opportunities require a large degree of financing over a long and uncertain timeline in order to exploit their potential – commonly hundreds of millions of dollars over a decade or more long horizon. Uncertainty arises from the often untested regulatory pathways [16], contested intellectual property portfolios, and difficulty in establishing clinical efficacy in the same manner as pharmaceuticals or devices.
Given the uncertainty, many funders are reluctant to invest in regenerative medicine. Historically, most stem cell therapy companies have failed. Among those that have undertaken initial public offerings (IPOs), most companies face stock prices that fall as much as 90% from their IPO levels [17].
The reluctance may be beginning to subside. BlueRock Therapeutics in Toronto and New York, received up to $225 million in Series A financing from Bayer and Versant Ventures in 2016, for instance. Investors appear to increasingly able to project market size, reimbursement rates, and regulatory paths as the first products have tested the waters. In turn, there has been substantial growth in frequency and value of acquisitions in the sector, signalling confidence in the market potential [18].
Nonetheless, despite the growing evidence of market support, there is still a substantial role for early stage research funding from public sources. Public financial support can help de-risk the progress made through academic scientific advances to a stage where the level of risk is acceptable to privately backed commercial organisations.
Many early stage funding programs have existed in many countries, offering real support for initial ventures. The oldest government-backed regenerative medicine institutions, the California Institute for Regenerative Medicine (CIRM), has stimulated a translationally focussed industry through grants with translationally focussed milestones. Two other key government-sponsored agencies are CCRM (Centre for Commercialization of Regenerative Medicine) in Canada and the Cell and Gene Therapy Catapult (CGTC) in the UK. These programmes act as incubators, allowing access to facilities or services with flexible arrangements for reimbursement, which could include taking a stake in a company. Flexible approaches not only in services or facilities rendered to clients, but also how these organisations are recompensed is an exemplar of the approach the field requires in order to overcome the complex challenges in advanced therapy development, manufacturing, regulation, and reimbursement [19].
A secondary role of such government supported organisations is to act as a champion for the field [19]. Competition in the field is intense not just between industries, but between geographic regions. The U.K. pioneered the field of monoclonal antibodies, yet lost out to the U.S. and Asia in translating these products, as the country lacked commercial infrastructure needed to develop and finance products to commercial scale. With cell therapy offering commercial potential on the same order as biologics, there are strong incentives to help generate needed infrastructure in support of local science.
Again, though, the tendency for each government to focus its funding and other support on a single country, or even local region within a country, risks missing the global span of funding and infrastructure needed to support commercialization. The sector could easily continue in the trap in which a scattered set of initiatives around the globe does not achieve needed coordination.
Intellectual property
Protection of inventions through patents and other intellectual property (IP) protection has long been viewed as essential to foster innovation in healthcare. In its simplest form, intellectual property protects an invention by facilitating an exclusive monopoly in the market for a set amount of time. In return, the inventor must disclose the fundamental nature of the invention so that when the protection expires the benefits may be more widely diffused. The overall goal is to provide a balance between reimbursement to inventors, incentives to continue to innovate rather than simply garner rents from prior innovations, and broad-based access.
To improve the current system of intellectual property protection it would be beneficial to emphasize transparency and consistency in the patent process globally. In an effort to fulfil these requirements the World Trade Organization (WTO) has worked towards an international agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS). This agreement outlines the minimal requirements for IP to enhance the compliance between WTO member states. Particular emphasis is paid to standards, enforcement, and dispute settlement for IP issues.
In regenerative medicine, the patent rights for CRISPR-Cas9 provide an example that emphasizes the need for clarity in claims when filing patent applications. The patent rights were initially filed by the University of California, Berkeley, indicating that CRISPR-Cas9 could be used to cut isolated DNA. This method was further advanced at the Broad Institute in Massachusetts, which highlighted the use of CRISPR-Cas9 to edit DNA in eukaryotic cells. Despite the earlier patent filing at Berkeley, the Broad Institute was granted patent rights first. The conflict between the institutions has led to uncertainty in the market and slowed commercial use of the technology [20].
The global scale of regenerative medicine research means that commercialization efforts need to engage with different IP regimes, despite the TRIPS efforts for uniformity. On a global scale, Japan and the U.S. are leaders in patent applications and granted patent rights, but have key differences in IP regimes. The life science patent environment in Japan limits patents granted to only those products that serve an industrial application, with medical activities and diagnostic methods for treatment on the human body not qualifying to be patentable. This raises contractual challenges when integrating key technologies across borders.
Commercial strategies
Production of regenerative medicine products diverges from those established for existing pharmaceuticals and biologics. Attempts to shoehorn these into existing frameworks have yielded poor outcomes. A classic exemplar is the immunotherapy Provenge, commercialized by Seattle’s Dendreon Corporation in 2010, which had a sound clinical product yet failed financially and led to the company’s bankruptcy [21].
Different regenerative medicine products necessitate varying manufacturing and business strategies. Broadly, there are two competing paradigms for production and sales: Centralised, where manufacturing capacity is located primarily in a few locations and one company takes the lead in marketing a product; and decentralised, where production units are geographically dispersed and multiple partners share marketing activities [22,23]. The centralised strategy has been the dominant production and sales scenario since the industrial revolution. Its key benefit is the reduction in costs through economies of scale. Conversely, decentralised strategies are relatively new scenarios that reflect the dispersion of specialized skills that are needed for different products and stages of development [24].
Part of the reason for Dendreon’s failure with Provenge stemmed from the company’s determination to undertake a centralized strategy when it lacked the skills needed for the full suite of activities in the market. Arguably, Dendreon would have had greater chance of success had it been willing to take a more decentralized approach, partnering with production and marketing allies around the world. Yet decentralised commercialization is more complex and requires substantially more coordination [21].
Complicating the split between the centralized and decentralized paradigms further is the question of logistics. The success of centralised manufacturing is directly tied to efficient logistical networks. Conversely, the strength of decentralised manufacturing is the ability to overcome logistical challenges of dispersion [25].
Some recent initiatives are providing logistical support for decentralized strategies. Figure 2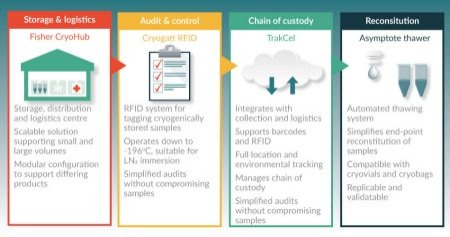
Another recent decentralization initiative arises in the form of scalable production sites, such as those developed at CCRM in Canada in partnership with GE Healthcare and at Loughborough University in the UK [27,28]. These facilities offer the promise of achieving lot sizes of several hundred billion to trillions of cells, needed for clinical testing and ultimately for market introduction. The facilities enable both economies of scale and reliably replicable production. Due to the wide variation in potential cell and gene therapies currently under scientific investigation, the manufacturing platforms of the future are likely to need to be developed as “process modules” that can be configured to suit the individual product being manufactured.
In parallel with the decentralized strategies, expansion by established pharmaceutical companies into regenerative medicine may be resulting in the use of more traditional centralized approaches. With the FDA’s approval of Novartis’s CAR-T therapy Kymriah and Gilead’s Yescarta (axicabtagene ciloleucel; obtained with Gilead’s acquisition of Kite Pharma in Los Angeles) in 2017, established pharmaceutical companies are becoming increasingly involved in the regenerative medicine market. As this engagement continues through a mix of internal development, collaborations, and acquisitions (e.g., Celgene’s acquisition of Seattle’s Juno Therapeutics in 2018), the participation helps validate the technologies and provides a strong base of regulatory and marketing skills. Even such cases, though, have strong decentralized aspects, involving partnerships across multiple countries to both develop and market the products.
DEMAND SIDE CHALLENGES: ACTORS IN THE MARKET
Regulatory policy
Navigating a product through the global regulatory pathway to market approval is a challenging task due to the diverse nature of requirements each state or region dictates. Additionally, a product will be regulated at differing levels that at first may not be clear to the researcher intently focussed on the technological aspect. Potential areas include product development, manufacturing, marketing and promotional activities, product labelling, and monitoring the lifecycle of the invention. While these regulatory roles are executed differently globally, they share the common goal of translating inventions and discoveries safely.
Closer global standardisation of regulatory policies would aid in market penetration of regenerative medicine products. Regulatory agencies have led the way in facilitating this market access with the progressive rollout of adaptive licensing schemes. However, this only supports product roll-out so far and additional integration would further simplify the development path for emerging therapies.
The European Union is an exemplar of harmonization of regulatory requirements, with the European Medicines Agency (EMA) acting as a decentralized agency for evaluating new products. Under this governance, companies submit an application to the EMA, which gives them the freedom of market authorization across the European Union as well as the additional countries that are members of the European Economic Area (Iceland, Liechtenstein and Norway).
Further convergence initiatives could be modelled on learnings from the Canadian Excellence in Clinical Innovation and Technology Evaluation (EXCITE) programme. This partnership was established in 2012 between government bodies, the health system, regulators, academia, clinicians, and industry partners with the goal of harmonizing the adoption of products, services, and technologies. The primary advantage of this initiative is quicker provisioning of emerging therapies to patients. In parallel, for regenerative medicine companies, the accelerated adoption process also fosters commercial growth.
Although more global regulatory convergence has been slow, in part due to the risk-averse nature of regulators, both firmer and more nuanced regulatory positions are emerging. Figure 3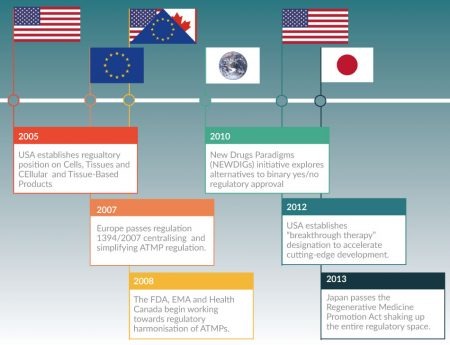
Reimbursement & health technology assessment
Market entry and expansion requires an effective reimbursement strategy. However, substantial variations in health technology assessment (HTA) and public and private reimbursement policies between countries and regions denies the potential for a global reimbursement strategy for an emerging regenerative medicine therapeutic. With rejection rates for medical technology adoption in the market running at 50%-95% even after approval, substantial market uptake is required for successful products. Fragmentation in reimbursement policies means that products will be introduced only in some markets or, in some cases, not introduced at all.
Reimbursement levels will never converge fully across countries due to multiple factors, including variation in local health needs and health priorities, as well as local politics and differences in ability to pay for expensive treatments. Nonetheless, greater consistency where feasible would facilitate successful commercialization of path-breaking clinical services, particularly in markets with compatible priorities. Closer convergence of bodies such as the U.K.’s National Institute for Health and Care Excellence (NICE), the Canadian Agency for Drugs and Technologies in Health (CADTH), Germany’s Institute for Quality and Efficiency in Healthcare (IQWiG), and the National High Authority for Health (HAS) in France, for instance, would simplify the commercial landscape. Progress on harmonisation of reimbursement likely presents a relatively greater challenge given the high level of uncertainty around the long-term efficacy of these advanced therapies. As the products become increasingly well characterised with regards to safety and efficacy, the appetite to simplify reimbursement may improve.
Multi-dimensional stakeholder engagement
The advanced therapeutics field is still evolving, and at this early stage it is imperative that key stakeholders become engaged. For instance, consider interactions among manufacturing, health technology assessment, regulation, and reimbursement. Due to the relative infancy of the field, often it is challenging to identify factors driving the efficacy of the product which in turn dramatically increases the challenge in establishing an optimal manufacturing strategy for a therapy, such as decentralised manufacturing of CAR-T therapies in a micro-factory in the clinic or rapid 3D bio-printing of replacement tissues. These unknowns further complicate both regulatory negotiations and the value proposition for reimbursement by public and private payers. These factors are strongly interrelated and it is only recently with encouraging efficacy data, strong clinical demand, and a clearer value proposition that early CAR-T therapies have indicated that real progress is being made [29].
Patient engagement
Along with cross talk between stakeholders, it is crucial to have patient engagement during the healthcare policy decision-making process. Patient engagement includes patients themselves, as well as their families and health care providers. These opinions can significantly improve how health policies are defined and executed. An example of such an organization is the independent not-for-profit Canadian organization, Clinical Trials Ontario, which has a team responsible for patient and public engagement in clinical trials. Similarly, the European Patient’s Academy (EUPATI) is an example of patient engagement that supports the involvement of patients during the R&D life cycle in association with regulatory agencies, health technology assessment bodies, ethics boards and the pharmaceutical industry.
We stress that rather than waiting to engage patients after a product is released, it is useful and even essential to have patient input during clinical trial design [30]. In the pharmaceutical industry, for instance, many companies have introduced roles for patient representatives to contribute to clinical trial advisory boards. Patient representatives can help shape which indications to focus on in initial trials and provide insight about delivery methods and other design questions.
Although immersing oneself in patient advocacy can offer relief from the burdens of disease as well as help champion direction of resources into under-funded areas [31], it is important that these vested interests do not skew strategic choices to the detriment to the broader field [32]. During the 2000s, there has been increasing pressure from patient advocacy groups to allow the right to access experimental treatments [33], which runs the risk of promoting unproven treatments that rely on the human emotion of hope [34] more than on evidence. Clearly, patient advocates are critically important but, like all contributors to the field, they face ethical obligations to benefit patients in the field as a whole [32].
OPPORTUNITIES GOING FORWARD
When examining the history of regenerative medicine, it is easy to become frustrated by the slow progress. The first companies in the field have struggled and failed. Yet it is telling that the core science and clinical practices commonly have been acquired following these failures and are often still available from another company. This suggests that the scientific approaches are fundamentally strong but lack viable market strategies to deliver the clinical and economic success they merit.
In the current global climate, the value proposition of regenerative medicine therapies has real and immediate importance to global healthcare markets and must be a priority consideration in the clinical translation process. A substantial number of clinical trials are reaching Phase III. Even with conservative estimates on progression through this challenging trials and regulatory processes, we should witness multiple regenerative medicine clinical products over the next five years. Moreover, the regenerative medicine trials market has spread globally, with increasing numbers of trials occurring outside North America or Europe [35].
A key issue, though, is that these successes are largely driven by technological push as opposed to market pull. This has resulted in scientifically successful products lacking the pre-emptive or concurrent appraisal of whether the knowledge can be translated into a marketable healthcare product [35]. As we move forwards, several profound changes should catalyse the roll out of these novel therapies.
In order to unlock the global potential regenerative medicine represents, greater emphasis must be placed on global coordination of both research and commercialization. This “local to global pivot” does not simply emphasise collaborating between universities across borders. Rather, a wide range of stakeholders in the regenerative medicine sector need to buy into enlarging the scope of collaboration.
Several current examples stand out.
- Tapping regional expertise for global research and development: consider CCRM in Canada. This government–academic–industry convergence point, which includes partners in Australia, Sweden the UK, and several other countries, acts much like the Cell Therapy Catapult in the UK in an effort to catalyse the translation of promising science to products to commercialisation. CCRM differs in its vision from the UK initiative, though, through its explicit strategy to make development pathways transnational.
- Regional replicability of global infrastructure: Several current initiatives are offering cross-border services for manufacturing and other infrastructural services. Progenitor Cell Therapies in New Jersey is a contract manufacturer that offers transnational penetration for manufacturing regenerative medicine products, including a facility in Japan. There has been increasing recent interest in the concept of such interconnected industrial environments, with terms such as “Smart Factories” (IBM), “Industrial Internet” (GE), “The Factory of the Future” (Airbus), and “Industrie 4.0” (German government) [36,37]. These concepts have the potential to provide next generation manufacturing [38], logistics and supply chain management [36], smart networks, automation [39] and big data [40] that represent a paradigm shift from traditional centralised manufacturing [41]. Critically, they facilitate replicable manufacturing and confidence in quality in manufacturing sites regardless of geography. This is a revolutionary catalyst for enabling the global regenerative medicine manufacturing value chain.
- Point to point global logistics: ThermoFisher Scientific’s CryoHub global network has the potential to relieve the logistical burden of cold chain services. The network highlights the recognition that even at the development/clinical trial manufacturing stage, global barriers are detrimental to bringing products to market. Technology and automation again has much to offer here, moving beyond the polystyrene dry-shipping box to smarter, lighter and fully trackable technologies has the potential to revolutionise the supply chain of advanced therapies.
- International standards: As therapies have progressed through trials and manufacturing scenarios, compliance typically has been enforced through local quality management systems that build on international guidelines. Whilst guidelines such as those provided by the International Society for Stem Cell Research (ISSCR) are useful in providing a framework to standardise innovative concepts, they are open to interpretation and are voluntary. Aspects of regenerative medicine manufacturing are currently governed by the International Organization for Standardization (ISO), but not the process itself. As the field has matured, the ability to standardise has become ever more possible. ISO standardisation is currently being agreed internationally; within the coming years, this will yield objectively measurable minimal standards defining the manufacturability of regenerative medicine products.
Converting the scientific potential of regenerative medicine technology into broad-based therapeutic value remains a significant medical and commercial challenge. The regenerative medicine field is still surrounded by a wide variety of scientific, technological, legal, and ethical issues that have slowed the path to commercial scale and clinical usage. If regenerative medicine science is to realize its full commercial potential, stakeholders throughout the sector will need to adopt novel coordination strategies to overcome these challenges.
WHO WILL COORDINATE?
The need for global coordination points raises a key final question: Who will be the coordinators? As the sector progresses, much of the “local to global pivot” is likely to be driven by industry, whether as consortia or a small number of lead firms, possibly in partnership with academic and public allies. Commercial players often are most likely to have both the skills and the incentives to undertake the wide-ranging strategies required to achieve successful coordination of the globally dispersed knowledge and activities that mark the regenerative medicine field.
Nonetheless, despite the clear coordination role for commercial vendors, there is still strong potential for academic/government (A/G) bodies to function as integrators, rather than simply exist as isolated entities at the periphery of the commercial ecosystem. A/G integrators have strong potential roles in shaping standards, building manufacturing skills, and many other aspects of physical and intellectual infrastructure needed to achieve successful commercialization and clinical use of regenerative medicine science. Such A/G integration is most likely to occur in partnerships with commercial vendors, typically with the A/G partners taking responsibilities for sub-systems within the commercial value chain.
Traditionally, partnerships with industry have been viewed with distrust by many academics and public actors. Yet, getting past the distrust and finding ways to align industry-academic-public incentives is a key part of the global commercialization pathway. Such successful relationships will both help A/G actors capture part of the commercial value of regenerative medicine science and, most importantly, smooth the pathway to successful translation into broad-based clinical value around the world.
ACKNOWLEDGEMENTS
Manuscript was prepared and written by RH and AG. Diagrams were prepared by RH. Feedback and further articulation for the article was provided by WM.
This study was supported by an EPSRC ETERM Landscape fellowship grant (RH) reference EP/I017801/1.
The development of this paper was influenced by the Summer by Design Program in Canada. Summer by Design is part of Medicine by Design at the University of Toronto. Medicine by Design is funded in part by the Canada First Research Excellence Fund (CFREF).
The authors would like to thank the valuable insights from participants sent by Loughborough University and The University of Nottingham (UK), Karolinska Institute (Sweden), Monash University and The University of Western Australia (Australia), Hannover Medical School (Germany), Erasmus Medical Centre and The Leiden University Medical Centre (Netherlands), National University of Singapore (Singapore), Kyoto University (Japan) and University of Toronto (Canada).
The authors would like to acknowledge the support of The Rotman School of Management, University of Toronto, Medicine by Design (MbD) and the CCRM (Centre for Commercialization of Regenerative Medicine) for their support in coordinating the expert meeting.
The draft of this document was reviewed by Patrick Bedford (Clinical Translation and Regulatory Affairs at CCRM). These contributions have aided in a number of revisions to clarify the subtle challenges faced in each aspect of the Regenerative Medicine field with the aim of increasing the utility of the document to the community.
The opinion reflected in this report is the opinion of the authors and their interpretation and aggregation of the opinion of the individual thought leaders as members of a selected expert reference panel. It does not represent the views of their employers or any organisations they may represent.
DISCLOSURE OF INTERESTS
RH now works for The Cell and Gene Therapy Catapult. The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
1. Kemp P. Regenerative medicine: looking backward 10 years further on. Regen. Med. 2016; 11(8): 787–800. CrossRef
2. Alliance for Regenerative Medicine. ARM Q3 2017. 2017; Quarterly Data Report.
3. Yin P. 3 Keys To Scale-Up CAR T-Cell Therapy Manufacturing. BioProcess Online 2017.
4. Heathman TRJ, Nienow W, Mccall MJ, Coopman K, Kara B, Hewitt CJ. 2015; <em10: 49–64.
5. MMIP. Advanced Therapies Manufacturing Action Plan; 2016.
6. UK BioIndustry Association. Building something great : UK’s Global Bioscience Cluster 2016. 2016.
7. Taylor P Astellas pledges $102.5M for universal donor cell company www.fiercebiotech.com/biotech/astellas-pledges-102-5m-for-universal-donor-cell-company (accessed Mar 15, 2018).
8. Gilead Buying Kite for $11.9 Billion. Cancer Discov. 2017; 7 (10): 1055–1056. CrossRef
9. GEN. Hitachi Chemical Agrees $75M Deal for Caladrius Cell Therapy Manufacturer, PCT www.genengnews.com/gen-news-highlights/hitachi-chemical-agrees-75m-deal-for-caladrius-cell-therapy-manufacturer-pct/81254041 (accessed Mar 15, 2018).
10. Miles I. Services innovation coming of age in the knowledge-based economy. Int. J. Innov. Manag. 2000; 4 (4): 371.
11. Valdivia W. University start-ups: critical for improving technology transfer. Center for Technology Transfer at Brookings 2013.
12. Phan PH, Decker A, Decker V (Eds). Academic Entrepreneurship: Translating Discoveries to the Marketplace. The Johns Hopkins University Series on Entrepreneurship. 2016. CrossRef
13. Rombach D. Proceedings of the 22nd international conference on Software engineering – ICSE ’00. ACM Press: New York, New York, USA, 2000; 531–537.
14. Written evidence submitted by The Work Foundation (TIC 56). Parliamentary Committees of Science and Technology 2010.
https://publications.parliament.uk/pa/cm201011/cmselect/cmsctech/619/619vw52.htm
15. Heinze T, Kuhlmann S. Across institutional boundaries?: Research collaboration in German public sector nanoscience. Res. Policy 2008; 37 (5): 888–899. CrossRef
16. Rosemann A, Bortz G, Vasen F, Sleeboom-Faulkner M. Global regulatory developments for clinical stem cell research: diversification and challenges to collaborations. Regen. Med. 2016; 11(7): 647–657. CrossRef
17. O’Brien JM. The great stem cell dilemma. Fortune.com http://fortune.com/2012/09/28/the-great-stem-cell-dilemma (accessed Jul 16, 2017).
18. Alliance for Regenerative Medicine. Annual Data Report on gene and cellular therapies and the regenerative medicine sector; 2016.
19. Schachter, B. Therapies of the state. Nat. Biotechnol. 2014; 32(8): 736–741. CrossRef
20. Ledford, H. News: Broad Institute wins bitter battle over CRISPR patents. Nature 2017; 542(7642): 401–401. CrossRef
21. Harrison RP, Ruck S, Rafiq QA, Medcalf N. Decentralised manufacturing of cell and gene therapy products: Learning from other healthcare sectors. Biotechnol. Adv. 2017; 36(2): 345–357. CrossRef
22. Medcalf, N. Centralized or decentralized manufacturing? Key business model considerations for cell therapies. Cell Gene Ther. Insights 2016; 2(1): 95–109.
23. Harrison RP, Ruck S, Medcalf N, Rafiq QA. Decentralized manufacturing of cell and gene therapies: Overcoming challenges and identifying opportunities. Cytotherapy 2017; 19(10): 1140–1151. CrossRef
24. Harrison RP, Rafiq QA, Medcalf N. Automating decentralized manufacturing of cell & gene therapy products. Cell Gene Ther. Insights 2016; 2(1): 115–120. CrossRef
25. Harrison RP, Medcalf N, Rafiq QA. Cell therapy-processing economics: small-scale microfactories as a stepping stone toward large-scale macrofactories. Regen. Med 2018; 13(2): 159-173. CrossRef
26. Cell and Gene Therapy Catapult. Fisher BioServices CryoHub to co-locate with manufacturing centre https://ct.catapult.org.uk/news-media/fisher-bioservices’-cryohub-co-locate-manufacturing-centre (Accessed Aug 5, 2017).
27. Rafiq QA, Brosnan KM, Coopman K, Nienow AW, Hewitt CJ. Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 2013; 35(8): 1233–1245. CrossRef
28. Eaker S, Abraham E, Allickson J et al. Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Cytotherapy 2017; 19(1): 9–18. CrossRef
29. Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther. Methods Clin. Dev. 2017; 4: 92–101. CrossRef
30. Smith SK, Selig W, Harker M et al. Patient engagement practices in clinical research among patient groups, industry, and academia in the United States: a survey. PLoS One 2015; 10 (10), e0140232. CrossRef
31. Dresser R. When Science Offers Salvation : Patient Advocacy And Research Ethics Oxford University Press 2001.
32. Mattingly TJ, Simoni-Wastila L. Patient-centered drug approval: the role of patient advocacy in the drug approval process. J. Manag. Care Spec. Pharm. 2017; 23(10): 1078-1082. CrossRef
33. Okie S. N. Engl. J. Med. 2006; 355 (5), 437–440. CrossRef
34. Editorial. FDA should stand firm on stem-cell treatments. Nature2016; 535 (7610), 7–8. CrossRef
35. Bubela T, McCabe C. Value-engineered translation for regenerative medicine: meeting the needs of health systems. Stem Cells Dev. 2013; 22(Suppl. 1): 89–93. CrossRef
36. Branke J, Farid SS, Shah N. Industry 4.0: a vision for personalized medicine supply chains? Cell Gene Ther. Insights 2016; 2 (22): 263–270. CrossRef
37. Slama D, Puhlmann F, Morrish J, Bhatnagar RM. Enterprise IoT: Strategies and Best Practices for Connected Products and Services. O’Reilly Media, Inc. CA, USA 2015.
38. Lee J, Bagheri B, Kao H-A. A cyber-physical systems architecture for industry 4.0-based manufacturing systems Manuf. Lett. 2015; 3: 18–23.
39. Jazdi N. In: 2014 IEEE International Conference on Automation, Quality and Testing, Robotics; IEEE 2014; 1–4.
40. Posada J, Toro C, Barandiaran I et al. Visual computing as a key enabling technology for Industrie 4.0 and Industrial Internet. IEEE Comput. Graph. Appl. 2015; 35(2): 26–40. CrossRef
41. Lipietz A. The post-Fordist world: labour relations, international hierarchy and global ecology. Rev. Int. Polit. Econ. 1997; 4(1): 1–41. CrossRef
Affiliations
Richard P Harrison
Centre for Biological Engineering, Holywell Park, Loughborough University, Loughborough, LE11 3TU, UK
Aileen Gracias
Department of Neuroscience, Karolinska Institute, 171 77, Stockholm, Sweden
William Mitchell
Rotman School of Management, University of Toronto, 105 St. George Street, Toronto, Ontario M5S 3E6
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</