Advances in Targeting CAR-T Therapy for Immune-Mediated Diseases
Cell Gene Therapy Insights 2018; 4(4), 255-265.
10.18609/cgti.2018.026
In recent years, genetically engineered cell therapies based on chimeric antigen receptor (CAR) technology have transformed the cancer treatment landscape. The groundbreaking success of CD19-specific CAR-T cell therapies in inducing lasting remissions of previously refractory B cell neoplasms has stimulated increasing interest in evaluating the potential of CAR technology to extend beyond cancer to induce safe and durable remissions of immunologic diseases. This review will highlight recent preclinical advances in targeting cytotoxic and regulatory CAR-T therapy to autoimmune and alloimmune disease indications, including pemphigus vulgaris, Factor VIII inhibitors, and HLA-mediated transplant rejection.
Submitted for Peer Review: Mar 27 2018 Published: May 14 2018
In 2017, the United States Food and Drug Administration (FDA) approved two genetically engineered cellular immunotherapies for the treatment of B cell leukemias and lymphomas, representing the first gene therapies approved in the United States, as well as the first therapies based on chimeric antigen receptor (CAR) technology. CARs consist of an extracellular antigen-binding domain, transmembrane domain, and a CD3ζ cytoplasmic domain, which induces T cell activation in an MHC-independent manner and directs potent cytolytic activity against cells expressing the antigen targeted by the CAR extracellular domain. Second and third generation CARs incorporate CD28 and/or CD137 intracellular costimulatory signaling domains, which have been shown to prevent T cell exhaustion and promote persistence of memory CAR-T cells [1]. The remarkable success of anti-CD19 CAR-T cell therapy [2-6] in effecting lasting cures of previously refractory B cell cancers has stimulated widespread interest in broadening of the applications of CAR technology beyond cancer to immunologic diseases.
Autoimmunity occurs when the immune system mistakenly attacks self, rather than foreign pathogens. Alloimmunity results from immunologic reactions to transplanted organs or protein replacement therapies, which can endanger their normal physiologic function. Current therapies for immune-mediated diseases largely depend on immunosuppressants that globally dampen immune responses. Chronic immune suppression is typically required to maintain disease control, increasing the risk of life-threatening infections and other complications over time. Thus, developing strategies to specifically eliminate pathogenic autoimmune or alloimmune reactions while sparing desirable immune functions represents the “holy grail” for therapy, but until recently, feasible strategies for such precision therapeutics have been unattainable. Due to the specificity and potency of CAR-mediated targeting, CAR technology offers the unprecedented opportunity to engineer the immune system to permanently correct its own mistakes. Here, we review recent preclinical data on the application of CAR technology to autoimmune and alloimmune disease indications.
Beyond CAR-T: “Double A” CAAR-T cells for autoimmune disease therapy
Autoimmunity represents a significant disease burden affecting up to 8% of the population, and its incidence is rising [7]. Pemphigus vulgaris (PV), an autoimmune blistering disorder that causes devastating and life-threatening denudation of the skin and mucous membranes, is a paradigm for antibody-mediated autoimmune disease due to the clear causative role of anti-desmoglein antibodies in inducing epithelial blisters [8]. Desmoglein 3 (Dsg3) autoantibodies directly interfere with desmoglein intermolecular adhesive interactions, disrupt desmosome assembly and/or disassembly pathways, and can modulate keratinocyte adhesion signaling pathways, in a complement- and Fc-independent manner [8,9]. Most anti-Dsg3 antibodies target the extracellular cadherin (EC) 1 and 2 domains [10], where residues important for Dsg3 trans- and cis-adhesion reside [11,12].
Many antibody-mediated diseases are treated with rituximab, an anti-CD20 monoclonal antibody (mAb) originally approved for B cell lymphoma [13]. Such an approach targets all B cells, causing toxicities from generalized immune suppression, but at the same time does not effectively eliminate all B cells, resulting in disease relapses that require repetitive rituximab infusions to regain disease control. In pemphigus, chronic rituximab therapy is associated with a 5.4% annualized risk of grade 3 or higher infectious adverse events [14] and a 1.3-1.9% lifetime risk of fatal infection [15,16]. Thus, the ideal therapy would target only the disease-causing autoimmune cells to avoid the risks of general immune suppression.
The striking long-term remissions of otherwise refractory B cell leukemias and lymphomas with anti-CD19 CAR-T therapy inspired us to re-engineer this powerful technology for targeted therapy of autoimmunity, using PV for preclinical proof of concept. Whereas CARs employ an antibody against a cell surface antigen to direct T cell cytotoxicity, a chimeric autoantibody receptor (CAAR) displays the autoantigen as the extracellular domain of the chimeric immunoreceptor to focus T cell cytotoxicity on the pathogenic autoimmune B cells, which in PV express Dsg3 autoantigen-specific B cell receptors (BCRs). The published preclinical data demonstrating the efficacy and safety of Dsg3 CAAR-T cells in inducing disease remission in a mouse model of PV has previously been reviewed [17,18].
Comparing CAAR-T therapy of autoimmunity with CAR-T therapy of cancer, there are both similarities and differences in the opportunities and challenges of translating these gene-engineered cellular immunotherapies to human clinical use. Taking clinical experience from anti-CD19 CAR-T trials in B cell leukemias and lymphomas as a precedent, CAAR-T therapy of autoimmunity offers similar opportunity for potent efficacy and long-term engraftment to provide durable disease remissions, a potential one-time curative treatment. CAAR-Ts offer the additional benefit of being highly targeted to the disease-causing B cell population, a precision medicine approach that would eliminate only the autoantigen-specific B cells while sparing the remainder of the B cell repertoire, thereby avoiding the risks of generalized immune suppression associated with current autoimmune disease treatments. Early relapses from anti-CD19 CAR-T therapy are often due to mutation or downregulation of CD19 on the cancer cell surface [19]. However, such escape mechanisms are unlikely in antibody-mediated disease, because downregulating the BCR would hinder B cell activation and hence maturation into an antibody-secreting cell, and BCR mutation to no longer bind the autoantigen would render the B cell irrelevant to disease. Thus, the BCR is an obligate marker of the disease-causing cell population in antibody-mediated diseases.
Regarding challenges, CAAR-T poses the novel clinical scenario in which the therapeutic will be infused into patients with pre-existing autoimmunity to the therapy. The pathogenic autoantibody population in PV is IgG4 [20,21], which typically does not fix complement and binds poorly to activating Fc-gamma receptors. In preclinical studies, Dsg3 CAAR-T cells were effective in eliminating anti-Dsg3 B cells, even in the presence of soluble anti-Dsg3 antibodies, both in vitro and in vivo. By testing Dsg3 CAAR function against polyclonal PV serum as well as a panel of anti-Dsg3 mAbs that target diverse epitopes with varied binding affinities, it was found that some antibodies inhibit, some have no apparent effect, and some potentiate CAAR-T cytolytic activity, the latter being associated with interferon-gamma release and CAAR-T proliferation. In addition, pulse-chase cell surface labeling studies with anti-Dsg3 mAbs indicate that CAAR synthesis continually replenishes CAAR molecules on the T cell surface. Based on the ability of CAAR-T cells to proliferate in response to soluble anti-Dsg3 antibodies and to synthesize new CAAR molecules, the in vitro data suggests that anti-Dsg3 antibodies should not prevent and may actually enhance CAAR-T function. Accordingly, in a PV hybridoma mouse model, Dsg3 CAAR T cells effectively eliminated anti-Dsg3 BCR+ cells, despite circulating polyclonal anti-Dsg3 antibodies that could have activated Fc-mediated clearance mechanisms in the NSG model, as has previously been described [22].
In contrast, a major toxicity of CAR-T therapy of cancer, cytokine release syndrome (CRS), may not apply to CAAR-T therapy of autoimmunity. CRS is closely correlated with tumor cell burden [23,24], and autoantigen-specific B cells usually comprise less than 1% of the total B cell repertoire [25]. CRS could theoretically occur if CAAR-T cells encountered strongly activating soluble antibody, but presumably these effects would be tempered by the mix of inhibitory and activating antibodies present in each individual patient.
Phase 1b CAAR-T clinical trials in pemphigus are expected to open in 2019, which will offer the exciting opportunity to determine the safety and curative potential of CAAR-T for autoimmune disease therapy.
Raising the bar: BAR T cells for alloantibody-mediated diseases
Similar to CAARs, BARs (B-cell antibody receptors) have been engineered to target Factor VIII (FVIII)-specific alloimmune B cells in hemophilia A by expressing the alloantigen as the extracellular domain of the chimeric immunoreceptor [26]. Hemophilia A is an X-linked bleeding disorder caused by mutations in FVIII, a critical component of the blood coagulation cascade that localizes to sites of vascular injury to allow Factor IXa protease to catalyze the activation of Factor X. The disorder can be treated with recombinant or plasma-derived FVIII replacement therapy; however, up to 30% of patients receiving FVIII develop an anti-drug alloantibody response that can inactivate or clear the replacement therapy and places the patient at risk of life-threatening bleeding events [27]. FVIII comprises domains A1, A2, B, A3, C1, and C2. The C2 domain is responsible for maintaining the serum half-life of FVIII via binding to von Willebrand factor, and in a mutually exclusive fashion, binds to phosphatidylserine on activated platelets and endothelial cells [28-30]. The A2 and to a lesser extent the A3 domain are responsible for Factor IXa binding [31-33]. Most inhibitory antibodies accelerate FVIII clearance, or block or delay clotting by targeting A2 and C2 domains of FVIII [34].
The standard of care in patients that develop neutralizing alloantibodies is immune tolerance induction [35], consisting of repetitive FVIII injection to eliminate or reduce inhibitory antibody titers, but this approach works for only 30-60% of cases and the cumulative expense for such an approach can cost millions per patient [36]. Alternatively, FVIII inhibitory antibodies can be bypassed by agents such as activated factor VII, activated prothrombin complex concentrate, or more recently, emicizumab, a bispecific antibody that bridges Factors IXa and X [37,38], but these approaches also require chronic and hence costly therapy [39]. A preliminary report has indicated that expression of immunodominant A2 or C2 FVIII epitopes in the extracellular domain of a BAR enables cytotoxic BAR-T cells to kill FVIII-specific B cells expressing IgM and IgG receptors that recognize the corresponding FVIII conformational epitopes [26]. BAR expressing cytotoxic T cells lysed C2- and A2-specific hybridomas in vitro and in vivo, resulting in inhibition of the antibody response to FVIII in vivo.
Engineering immune tolerance: CAR and BAR regulatory T cells
An additional strategy for applying CAR technology to the treatment of immune-related disease is to genetically engineer regulatory T cells that can more broadly suppress immune responses, rather than cytotoxic T cells that specifically lyse the targeted cells. Regulatory T cells (Tregs) are a subset of CD4+ T cells that are most commonly defined by their high expression levels of interleukin (IL-2) receptor α chain CD25 and the transcription factor FOXP3. Tregs play an essential role in maintaining self-tolerance and have been shown to suppress both humoral and cellular immune responses toward self-antigens [40,41]. Moreover, many autoimmune diseases show a dysfunction in Tregs [42], and both mice and humans with nonfunctional Tregs develop autoimmunity [43,44]. These data on the critical role of Tregs in preventing autoimmunity underlie the rationale for engineering Tregs for therapeutic benefit. Antigen-specific Tregs should have increased potency when compared to polyclonal Tregs because of their ability to be directed toward desired antigens, meaning that fewer antigen-specific Tregs would be needed to achieve the same suppressive effect and could lower the risk of off-target suppression [45]. However, endogenously occurring antigen-specific Tregs are extremely rare, can be difficult to isolate, and need to undergo significant in vitro expansion to reach therapeutically relevant doses, which is challenging to achieve without loss of specificity and function [46]. Thus, using chimeric immunoreceptors to engineer antigen specificity into Tregs is a novel approach to overcome these limitations.
Previously, mouse Tregs were engineered to express antibody-based CARs specific for the hapten 2,4,6-trinitrophenol (TNP) [47,48]. These anti-TNP-CAR Tregs were shown to mediate antigen-specific suppression of effector T cells in vitro, and resistance to colitis in a dose-dependent manner in vivo. Similarly, mouse CAR Tregs specific for another model antigen, carcinoembryonic antigen (CEA), have been shown to home to the location of autoimmune activity and suppressed the severity of disease in a colitis disease model [49]. In addition, CARs specific for myelin oligodendrocyte glycoprotein (MOG) have been developed [50]. MOG is a pathogenic autoantigen for the induction of experimental autoimmune encephalomyelitis (EAE), which serves as a disease model for multiple sclerosis (MS), although a wider array of autoantigens has been implicated in human MS [51-53]. Mouse T cells were engineered to ectopically express FOXP3 to enforce the Treg phenotype as well as an anti-MOG CAR. Anti-MOG CAR Tregs suppressed anti-CD3/IL-2-stimulated T cell expansion in vitro and localized to the brain after intranasal delivery, associated with diminished symptoms of EAE in vivo. Collectively these studies provided proof-of-concept for CAR Tregs as a therapeutic strategy that could be further developed for human immune diseases.
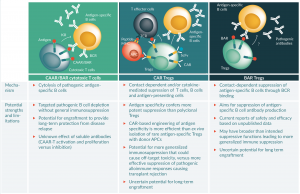
Figure 1: Schematic representation of the applications of chimeric immunoreceptor technology in immune-mediated diseases.
Preclinical studies of gene-engineered Tregs have explored their therapeutic potential to induce transplant and Factor VIII inhibitor tolerance. HLA-A2 is a commonly mismatched antigen in transplantation, and HLA-A mismatching is associated with poor outcomes after transplantation [54]. Tregs expressing antibody-based CARs specific for HLA-A*02:01 (HLA-A2) were evaluated for their ability to regulate alloreactive T cells that can cause rejection in hematopoietic stem cell transplantation (HSCT) and solid organ transplantation [55-57]. Use of CARs to direct antigen-specific Treg activity avoids problems associated with enriching antigen-specific Tregs ex vivo, including the rarity of antigen-specific Tregs and consequent need for significant expansion, which may compromise subsequent Treg survival, as well as the requirement for donor antigen-presenting cells. Anti-HLA-A2 CAR Tregs demonstrated increased proliferation when stimulated via the CAR as compared to the endogenous T cell receptor (TCR), maintained their expected phenotype and suppressive function despite the relatively strong CAR-mediated activation and expansion, and did not exhibit significant cytolytic activity. CAR-stimulated Tregs were significantly better at preventing xenogeneic GVHD in a humanized mouse model than Tregs that only received stimulation through the endogenous TCR [55]. Additional studies have demonstrated that anti-HLA-A2-CAR Tregs suppress alloimmune responses better than polyclonal Tregs, or Tregs expressing an anti-HLA-A2 CAR without the intracellular signaling domain (ΔCAR) in humanized mouse models of HLA-A2+ skin xenografts [56,57]. Off-target immune suppression with CAR Tregs may be lower when compared to polyclonal Tregs, because on-target efficacy can be achieved with smaller numbers of CAR Tregs, and Treg suppression was shown to be contact-dependent rather than through a general bystander effect. Overall, the published data suggests that targeting MHC class I alloreactivity with CAR Treg therapy could be a promising approach for transplant rejection, although additional methods may be necessary to address the humoral and cellular components of the alloimmune response to the full scope of MHC I and MHC II alleles that mediate transplant rejection. An IND filing for anti-HLA-A2 CAR Tregs is planned for late 2018.
Recent studies have also applied BAR and CAR technology to induce Treg tolerance for hemophilia A inhibitors. Tregs were engineered to be antigen-specific by expressing a Factor VIII alloantigen-based BAR [58] or an anti-FVIII CAR (ANS8 CAR) [59]. Alloantigen-expressing FVIII BAR Tregs have been reported to prophylactically suppress the antibody response to FVIII when injected into FVIII-deficient mice [58], an approach that aims for targeted suppression of FVIII inhibitor production by B cells. Broader targeting strategies have employed ANS8, an scFv antibody fragment that recognizes the A2 domain of FVIII. ANS8 CAR-transduced cells recognized free FVIII but more effectively proliferated to membrane or plate bound FVIII. ANS8 CAR Tregs not only suppressed the proliferation of FVIII-specific T effector cells, but also suppressed antibody responses to FVIII both in vitro and in vivo. Suppression of the humoral immune response by ANS8 CAR Tregs in vivo lasted up to 8 weeks, but the suppression was lost after re-challenge with FVIII, presumably due to rejection of the human Tregs. ANS8 CAR Tregs, despite being targeted toward the FVIII A2 domain, were also able to suppress FVIII C2-specific T effector cell proliferation in the presence of full-length FVIII, as well as proliferation of T effector cells targeted against the irrelevant antigen myelin basic protein (MBP) in the presence of MBP and FVIII [59]. Thus, despite antigen targeting through the CAR, in these studies genetically engineered Tregs were shown to exert more broadly immunosuppressive effects, also known as bystander or linked suppression [60]. In the context of transplantation rejection and hemophilia A, bystander suppression might also be beneficial by suppressing a broader array of MHCI- and MHCII-targeted T and B cell responses after HLA-mismatched transplantation, or could induce tolerance toward large proteins such as FVIII that otherwise might not be targetable through a single BAR.
Translational Insight
In summary, novel strategies utilizing chimeric immunoreceptor technology in cytotoxic and regulatory T cells have recently shown promising preclinical results for immune-mediated diseases. CAAR and BAR cytotoxic T cells aim for targeted B cell depletion of the pathogenic autoimmune or alloimmune B cell population, without risking the generalized immune suppression caused by total B cell depletion. CAR Tregs utilize an antibody against a target antigen to suppress immune responses at the site of antigen expression, whereas BAR Tregs express the alloantigen itself to suppress alloantigen-specific B cell responses. The next decade will offer exciting opportunities to bring these novel technologies forward to human clinical trials to determine whether CAR-T based approaches can offer safe and lasting remissions for immune-mediated diseases.
FINANCIAL & COMPETING INTERESTS DISCLOSURE
This work was supported in part by R01-AR068288 (ASP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ASP is a co-founder of Tycho Therapeutics, Inc., focused on targeted therapy of autoimmunity and is an inventor on patents licensed to Tycho Therapeutics and Novartis. No writing assistance was utilized in the production of this manuscript.
References
1. Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature 2017; 545(7655): 423–431.
CrossRef
2. Schuster SJ, Svoboda J, Chong EA et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017; 377(26): 2545–2554.
CrossRef
3. Turtle CJ, Hay KA, Hanafi LA et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Rece After Failure of Ibrutinib. J. Clin. Oncol. 2017; 35(26): 3010–3020.
CrossRef
4. Kochenderfer JN, Somerville RPT, Lu T et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Mol. Ther. 2017; 25(10): 2245–2253.
CrossRef
5. Park JH, Rivière I, Gonen M et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018; 378(5): 449–459.
CrossRef
6. Bollard CM, Tripic T, Cruz CR et al. Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. J. Clin. Oncol. 2018: JCO2017743179.
CrossRef
7. National Institutes of Health Report of the Director 2012. National Institutes of Health.
8. Kasperkiewicz M, Ellebrecht CT, Takahashi H et al. Pemphigus. Nat. Rev. Dis. Primers 2017; 3: 17026.
CrossRef
9. Spindler V, Eming R, Schmidt E et al. Mechanisms Causing Loss of Keratinocyte Cohesion in Pemphigus. J. Invest. Der-matol. 2018; 138(1): 32–37.
CrossRef
10. Ohyama B, Nishifuji K, Chan PT et al. Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudi-nal study using desmoglein 2-based swapped molecules. J. Invest. Dermatol. 2012; 132(4): 1158–1168.
CrossRef
11. Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 2002; 296(5571): 1308–1313.
CrossRef
12. Harrison OJ, Brasch JL Lasso G et al. Structural basis of adhesive binding by desmocollins and desmogleins. Proc. Natl Acad. Sci. USA 2016; 113(26): 7160-7165.
CrossRef
13. Ran NA, Payne AS. Rituximab therapy in pemphigus and other autoantibody-mediated diseases. F1000Res 2017; 6: 83.
CrossRef
14. Joly P, Maho-Vaillant M, Prost-Squarcioni C et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label ran-domised trial. Lancet 2017; 389(10083): 2031–2040.
CrossRef
15. Hans-Peter T, Burmester G, Schulze-Koops H et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res. Ther. 2011; 13(3): R75.
CrossRef
16. Wang HH, Liu CW, Li YC, Huang YC et al. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm. Venereol. 2015; 95(8): 928–932
CrossRef
17. Amagai M. Modulating Immunity to Treat Autoimmune Disease. N. Engl. J. Med. 2016; 375(15): 1487–1489.
CrossRef
18. Ellebrecht CT, Payne AS. Setting the target for pemphigus vulgaris therapy. J. CI. Insight. 2017; 2(5): e92021.
19. Ruella M, Maus MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput. Struct. Biotechnol. J. 2016. 14: p. 357-362.
CrossRef
20. Funakoshi T, Lunardon L, Ellebrecht CT, Nagler AR, O’Leary CE, Payne AS. Enrichment of total serum IgG4 in pa-tients with pemphigus. Br. J. Dermatol. 2012; 167(6): 1245–1253.
CrossRef
21. Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J. Dermatol. Sci. 2001; 26(1): 55–61.
CrossRef
22. Jonnalagadda M, Mardiros A, Urak R et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol. Ther. 2015; 23(4): 757–768
CrossRef
23. Fitzgerald JC, Weiss SL, Maude SL et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit. Care Med. 2017; 45(2): e124-e131.
CrossRef
24. Hay KA, Hanafi LA, Li D et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017; 130(21): 2295–2306.
CrossRef
25. Nishifuji K, Amagai M, Kuwana M, Iwasaki T, Nishikawa T et al. Detection of antigen-specific B cells in patients with pemphigus vulgaris by enzyme-linked immunospot assay: requirement of T cell collaboration for autoantibody produc-tion. J. Invest. Dermatol. 2000; 114(1): 88–94.
CrossRef
26. Parvathaneni, KZ, Kim A, Scott YC. BAR-CD8 T-Cell Mediated Targeted Killing of Inhibitor Producing FVIII-Specific B Cells. Blood 2015.
27. Peyvandi F, Mannucci PM, Garagiola I et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemo-philia A. N. Engl. J. Med. 2016; 374(21): 2054–2064.
CrossRef
28. Foster PA, Fulcher CA, Marti T, Titani K, Zimmerman TS et al. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von Willebrand factor. J. Biol. Chem. 1987; 262(18): 8443–8446.
29. Saenko EL, Shima M, Rajalakshmi KJ, Scandella D. A role for the C2 domain of factor VIII in binding to von Willebrand factor. J. Biol. Chem. 1994; 269(15): 11601–11605.
30. Scandella D, Gilbert GE, Shima M et al. Some factor VIII inhibitor antibodies recognize a common epitope correspond-ing to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood 1995; 86(5): 1811–1819.
31. Fay PJ, Beattie T, Huggins CF, Regan LM et al. Factor VIIIa A2 subunit residues 558-565 represent a factor IXa interac-tive site. J. Biol. Chem. 1994; 269(32): 20522–20527.
32. Zhong D, Saenko EL, Shima M, Felch M, Scandella D. Some human inhibitor antibodies interfere with factor VIII binding to factor IX. Blood 1998; 92(1): 136–142
33. Amano K, Sarkar R, Pemberton S, Kemball-Cook G, Kazazian HH Jr, Kaufman RJ. The molecular basis for cross-reacting material-positive hemophilia A due to missense mutations within the A2-domain of factor VIII. Blood 1998; 91(2): 538–548″ rel=”noopener” target=”_blank”>CrossRef
34. Spiegel PC JR Jacquemin M, Saint-Remy JM, Stoddard BL, Pratt KP. Structure of a factor VIII C2 domain-immunoglobulin G4kappa Fab complex: identification of an inhibitory antibody epitope on the surface of factor VIII. Blood 2001; 98(1): 13–19.
CrossRef
35. Kasper CK, Pool JG. Letter: Measurement of mild factor VIII inhibitors in Bethesda units. Thromb. Diath. Haemorrh. 1975; 34(3): 875–876.
36. Earnshaw SR, Graham CN, McDade CL, Spears JB, Kessler CM. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia 2015; 21(3): 310–319.
CrossRef
37. Turecek PL, Váradi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia 2004; 10(Suppl 2): 3–9.
CrossRef
38. Uchida N, Sambe T1, Yoneyama K et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood 2016; 127(13): 1633–1641.
CrossRef
39. Rosenberg J. ICER Report: Despite High Price, Emicizumab for Hemophilia A Cuts Costs. 2018.
40. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012; 30: 531–564
CrossRef
41. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017; 17(11): 703–717.
CrossRef
42. Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000; 43(3): 617–627
CrossRef
43. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299(5609): 1057–1061.
CrossRef
44. Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat. Rev. Endocrinol. 2010; 6(5): 270–7.
CrossRef
45. Dawson NAJ, Vent-Schmidt J, Levings MK. Engineered Tolerance: Tailoring Development, Function, and Antigen-Specificity of Regulatory T Cells. Front. Immunol. 2017; 8: 1460.
CrossRef
46. Hoffmann P, Boeld TJ, Eder R et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009; 39(4): 1088–1097.
CrossRef
47. Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of exper-imental colitis in mice. Gastroenterology 2008; 134(7): 2014–2024.
CrossRef
48. Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redi-rected by antigen-specific chimeric receptor. Gastroenterology 2009; 136(5): 1721–1731.
CrossRef
49. Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoem-bryonic antigen-specific regulatory T cells. Mol. Ther. 2014; 22(5): 1018–1028.
CrossRef
50. Fransson M, Piras E, Burman J et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation 2012; 9: 112.
CrossRef
51. Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the develop-ment of multiple sclerosis. J. Clin. Invest. 1997; 99(7): 1682–1690.
CrossRef
52. Tuohy VK, Yu M, Yin L, Kawczak JA, Kinkel PR. Regression and spreading of self-recognition during the development of autoimmune demyelinating disease. J. Autoimmun. 1999; 13(1): 11–20.
CrossRef
53. Sospedra M, Martin R, Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005; 23: 683–747.
CrossRef
54. Park M, Seo JJ. Role of HLA in Hematopoietic Stem Cell Transplantation. Bone Marrow Res 2012; 2012: 680841.
CrossRef
55. MacDonald KG, Hoeppli RE, Huang Q et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016; 126(4): 1413–1424.
CrossRef
56. Boardman DA, Philippeos C, Fruhwirth GO et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am. J. Transplant. 2017; 17(4): 931–943.
CrossRef
57. Noyan F, Zimmermann K1, Hardtke-Wolenski M et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am. J. Transplant. 2017; 17(4): 917–930.
CrossRef
58. Zhang AHP, Yoon K, Kim JY Scott DW. Targeting antigen-specific B cells using BAR-transduced cytotoxic and regula-tory T cells. J. Immunol. 2016.
59. Yoon J, Schmidt A, Zhang AH, Königs C, Kim YC1, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood 2017; 129(2): 238–245.
CrossRef
60. Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspe-cific. J. Immunol. 2000; 164(1): 183–190
CrossRef
Affiliations
Jinmin Lee & Aimee S. Payne*
Department of Dermatology, University of Pennsylvania, Philadelphia, PA USA.
* Author for correspondence: aimee.payne@uphs.upenn.edu
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</

