Process development considerations for cryopreservation of cellular therapies
Cell & Gene Therapy Insights 2019; 5(9), 1151-1167.
10.18609/cgti.2019.122
This article discusses the biopreservation steps as part of the manufacturing process, and reviews what considerations should be part of the picture when incorporating cryopreservation. It will also review Biopreservation Best Practices recommendations for the cryopreservation step through two case studies – one using a human T cell model and the second, human donor T cells.
The role of cryopreservation in cell and gene therapy manufacturing
Figure 1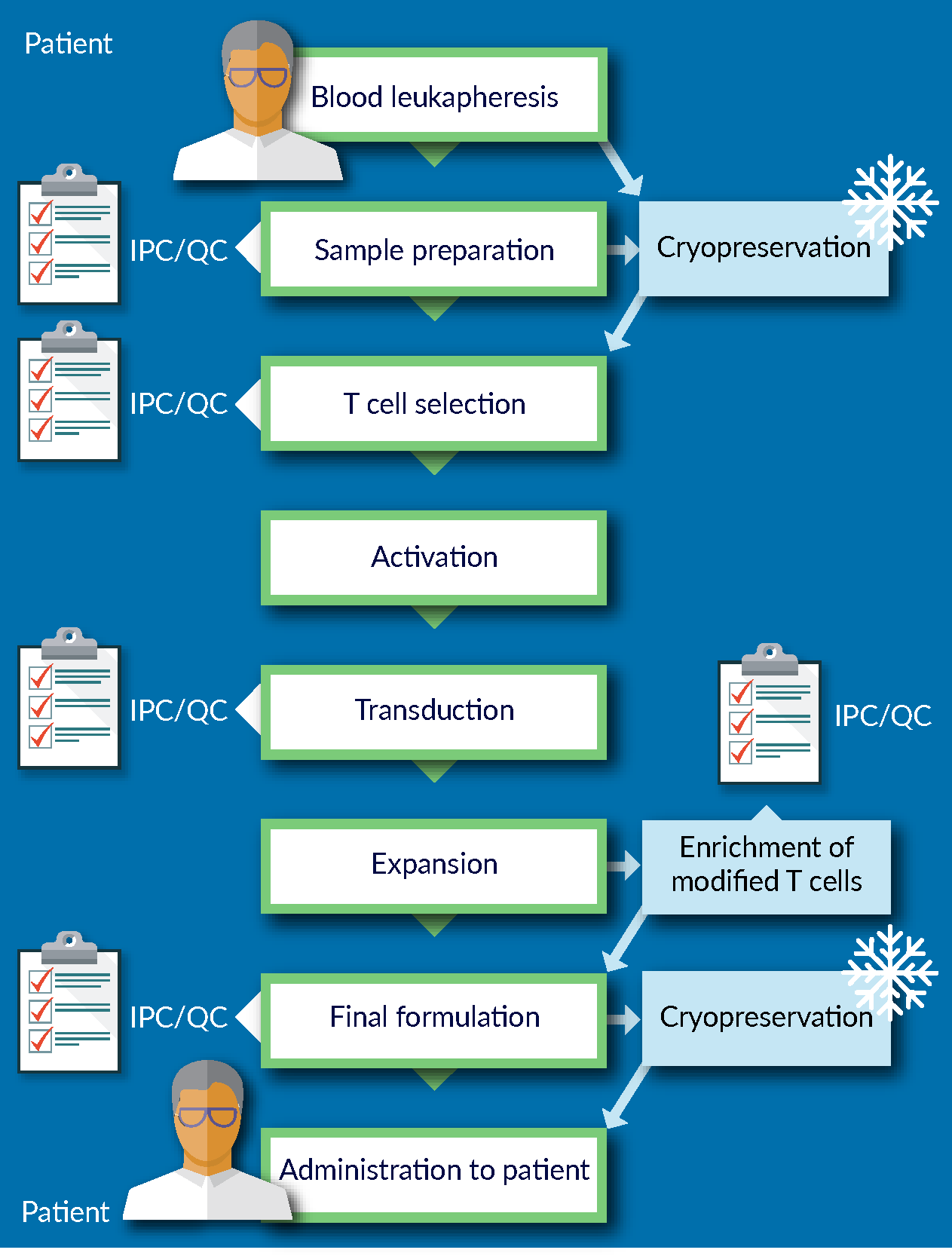
We are familiar with the fact that biological cells deteriorate over time, even in normal conditions. When outside of normal conditions, this deterioration process accelerates significantly. To date, the only process that is capable of arresting this biological degradation is cryopreservation.
Cryopreservation is the process of lowering the temperature of the biological system to below -130°C, at which point the water-based biological system will be below the glass transition temperature, molecular motion will be arrested and the entire system will be in a state of “suspended animation”.
This concept theoretically allows for indefinite storage of biological materials. It buys the manufacturer time to conduct all necessary assays and prepare documentation for release. It also allows for more robust, reliable transport and shipping options. Today, the availability of shippers that are able to maintain the temperature of -130°C or below for days at a time, offer a considerable degree of convenience and flexibility over non-frozen cell products shipping.
Before implementing the cryopreservation step as part of the process, development work needs to be performed to ensure that cryopreservation is not adversely impacting the starting material entering the manufacturing process: the robustness of the manufacturing process depends in large part on the quality of the starting material. Similarly, the quality of the final product could also be significantly affected if cryopreserved. In other words, cryopreservation is the first AND the last step of the manufacturing process, and therefore, it is crucial to minimize the impact of cryopreservation on process and product.
Traditionally, the approach to cell preservation in academic and research environment is to formulate the cells in what is commonly known as a “home-brew” – a mixture of culture media with various amounts of human or animal serum, and different concentrations of dimethyl sulfoxide (DMSO). Once in this formulation, the cells are then typically stored at -80°C overnight before transferring to LN2 storage. A recovery rate of 50% of cells may be perfectly acceptable to a researcher. However, that is not the case when one is developing a cellular therapy product. The advent of tissue engineering and regenerative medicine has led to more stringent quality and regulatory considerations for therapies that could be considered first-line of treatments for patients.
Certain best practices are recommended across the field of cell and gene therapy. Some of these best practices are based on risk mitigation – for example, using a GMP-manufactured, fully-defined media, whether it be a culture media in upstream processing for vector manufacturing, or downstream processing, or in the cryopreservation step. Essentially, using a fully-defined media versus a non-fully-defined media reduces a lot of the risk associated with process changes. It increases control over the process and facilitates conducting failure mode and error analysis (FMEA).
Another example of best practice for cryopreservation is avoiding a wash and reformulation step after thawing. This may be achieved by qualifying the cryopreservation reagent as an excipient rather than as an ancillary material. One could consider having a wash and reformulation step if the components of cryopreservation media are not approved for human administration. In that case, equipment, trained personnel and potentially a cleanroom facility either at or near to the patient’s bedside are required to conduct the wash and reformulation process. This effectively extends the manufacturing process beyond the central bioprocessing site on the point of care, which would be counterintuitive to having a centralized manufacturing model. The requirement for additional equipment and trained labor would also add significantly to the costs of manufacturing and increases risks and process and product variability. As such, designing a process that eliminates the wash and reformulation step after cryopreservation is highly recommended.
Risk mitigation in cryopreservation
An example of risk mitigation is the use of intracellular-like media. Before delving more into this point, the advantages of using an intracellular-like media for cryopreservation are reviewed below. On the left of Figure 2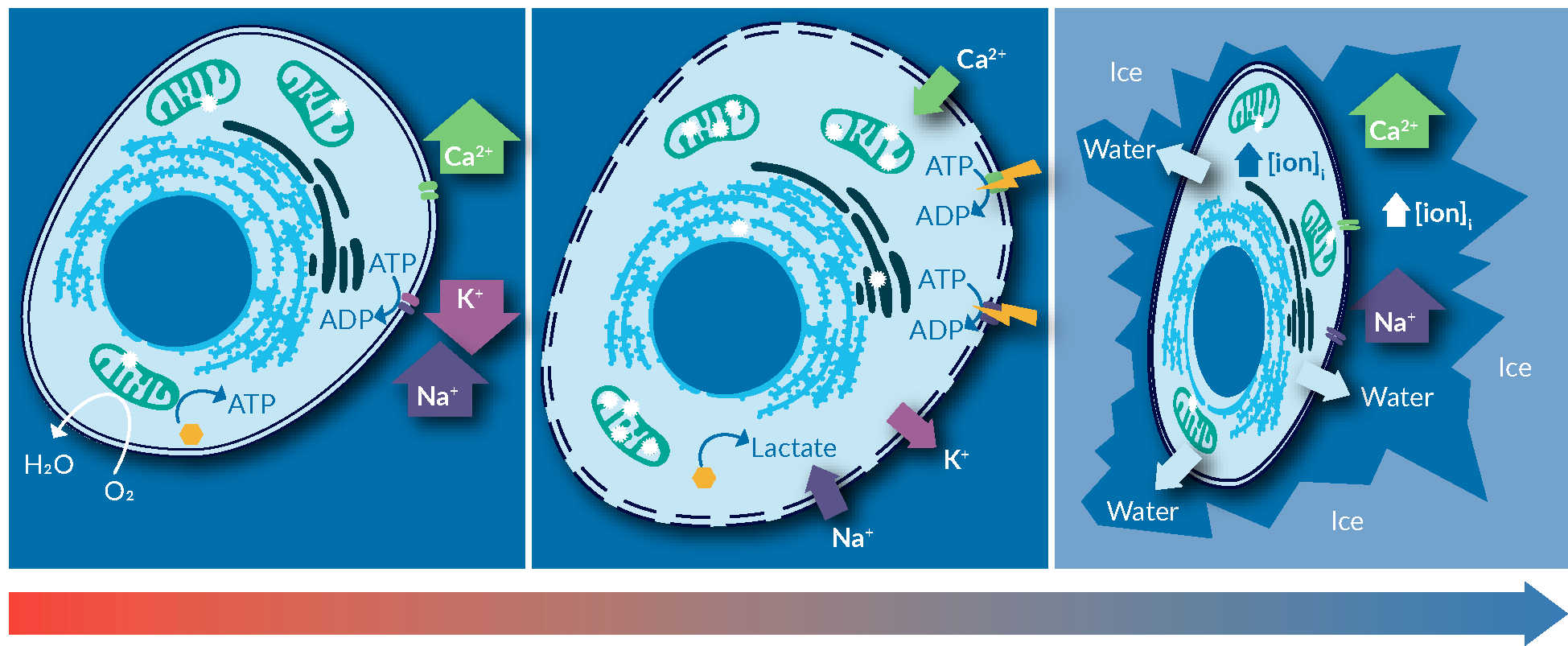
Cold-induced membrane permeabilization the freezing point of the solution, is exacerbated below, where ice formation concentrates the solutes in the solution. Published measurements suggest that at -20°C, ice formation increases the concentration of salts in the solution to as high as 20-times the norm-osmotic concentrations. This significantly increases the toxicity of freezing media containing salts on cells, through changes in salinity and pH of the intracellular milieu. Upon thawing, cells experience a completely imbalance of intracellular ionic strength, disrupted intracellular signaling, and a significant load of misfolded or denatured proteins. The extent of stress and freezing-induced damages can overwhelm the capacity of intracellular machinery to repair and recover, as such inducing apoptosis mechanism in cells. The major reason of observed post-thaw cell loss.
An easy fix for this problem, one that is applicable to most cell types, is to use a solution that mimics the intracellular ionic balance. This can minimize the gradient of ions across the cell membrane, hence, reducing cold-induced cellular stresses during the freezing process.
An easy fix for this problem, one that is applicable to most cell types, is to use a solution that mimics the intracellular environment. This eliminates the gradient of ions across the cell membrane, removing all of those stresses exerted on cells during the freezing process.
However, it is to be noted that most evidence-based best practices are process dependent. The use of intracellular- versus extracellular-like media is a general recommendation, T cells, NK cells or stem cells, have different biology and different susceptibility to cold, to sodium or ionic strength in the media, and different mechanical response to shrinking and swelling induced by the freezing and thawing processes. Furthermore, the specific steps applied between harvest and thaw may impact the cells differently. The suggestion is therefore to identify all the steps in the cryopreservation process and all the parameters of each step to examine the criticality of each step of the process. A few of the parameters for each potential step are listed in Figure 3
It is imperative to begin at a very early stage of cell therapy development to think about how the final product is going to reach the patient. Whether the final product is in liquid or frozen form may have serious implications for the overall business plan and manufacturing model: a centralized manufacturing model involving shipping a frozen product, or a more decentralized or localized manufacturing model allowing for a ‘fresh’ product to reach patients sufficiently quickly, given its shorter shelf-life, each with significantly different requirements in terms of capital expenditure, staffing, quality and regulatory foot print, to name a few.
Case studies
To illustrate the criticality of the process parameters, we will discuss two cases studying the impact of proper freezing media. The first is on functional assessment of the impact of cryopreservation on human CD3 T cells, and the second is on identifying critical process parameters of cryopreservation using a T cell model (Jurkat cells).
Functional assessment of the impact of cryopreservation on human CD3 T cells
The first case study was selected to illustrate the impact of intracellular- versus extracellular-like media formulation and DMSO content, and how it impacts the critical quality attributes (CQAs) of the product. This case study also illustrates the impact of two process parameters relevant to manufacturing process design, namely thawing and delivery model, and post-thaw stability time frame. Post-thaw stability is particularly are a topic of much conjecture: how stable is the product between thawing and administration to the patient before it begins to lose potency? A further common question regarding the starting material is, what is the best strategy to incorporate cryopreserved starting material into the manufacturing process? Do the thawed cells need to ‘rest’ for a period of time before activation and transduction, or should they enter this process immediately? The answer to this question may have a significant impact on the manufacturing process timeline and efficiency.
In this study, human Pan CD3 T cells were obtained from HemaCare. The primary goal was to explore the impact of serum elimination and use of intracellular media from the cryopreservation media. For this purpose, four different media formulations were compared head to head: traditional home-brew formulations using Normosol R or PlasmaLyte-A, together with 5% w/w recombinant human serum albumin, and 10% v/v DMSO. Both of these formulations are very commonly used across publications in the cell therapy field. (In fact, one of these formulations is currently used in an approved product). The performances of these formulations were compared with those of intracellular-like media, which is the base formulation of CryoStor media. CryoStor CS5 and CryoStor CS10 were used, both of which are devoid of serum and protein, but are instead formulated with sugars and other macromolecules at a similar weight per weight percentage. (CS5 has 5% DMSO and CS10 has 10% DMSO).
Human frozen down CD3 T cells were thawed in ImmunoCult T cell culture media and immediately activated using ImmunoCult CD3/CD28/CD2 soluble activation agent (both reagents from (STEMCELL Technologies). The cells were then expanded after 24 hours of stimulation by addition of ImmunoCult media. Upon reaching desired cell count after 9 days, the cells were harvested and formulated in 4 different cryopreservation media, vialed and cryopreserved using a liquid nitrogen-free controlled rate freezer. After transfer to, and a minimum of overnight storage in, liquid nitrogen, the samples were thawed in fresh culture media and placed back into culture. Each sample was divided into two: a control group rested in media supplemented with IL-2, another group rested in media supplemented with IL-2 plus ImmunoCult™ – an anti-CD3/CD28/CD2 activation agent from STEMCELL Technologies.
The cells were followed post-thaw for 3 days. They were assayed for viability, count, and INF-γ secretion on day zero (i.e. immediately post-thaw), at 24 hours and at 72 hours post-thaw.
Figure 4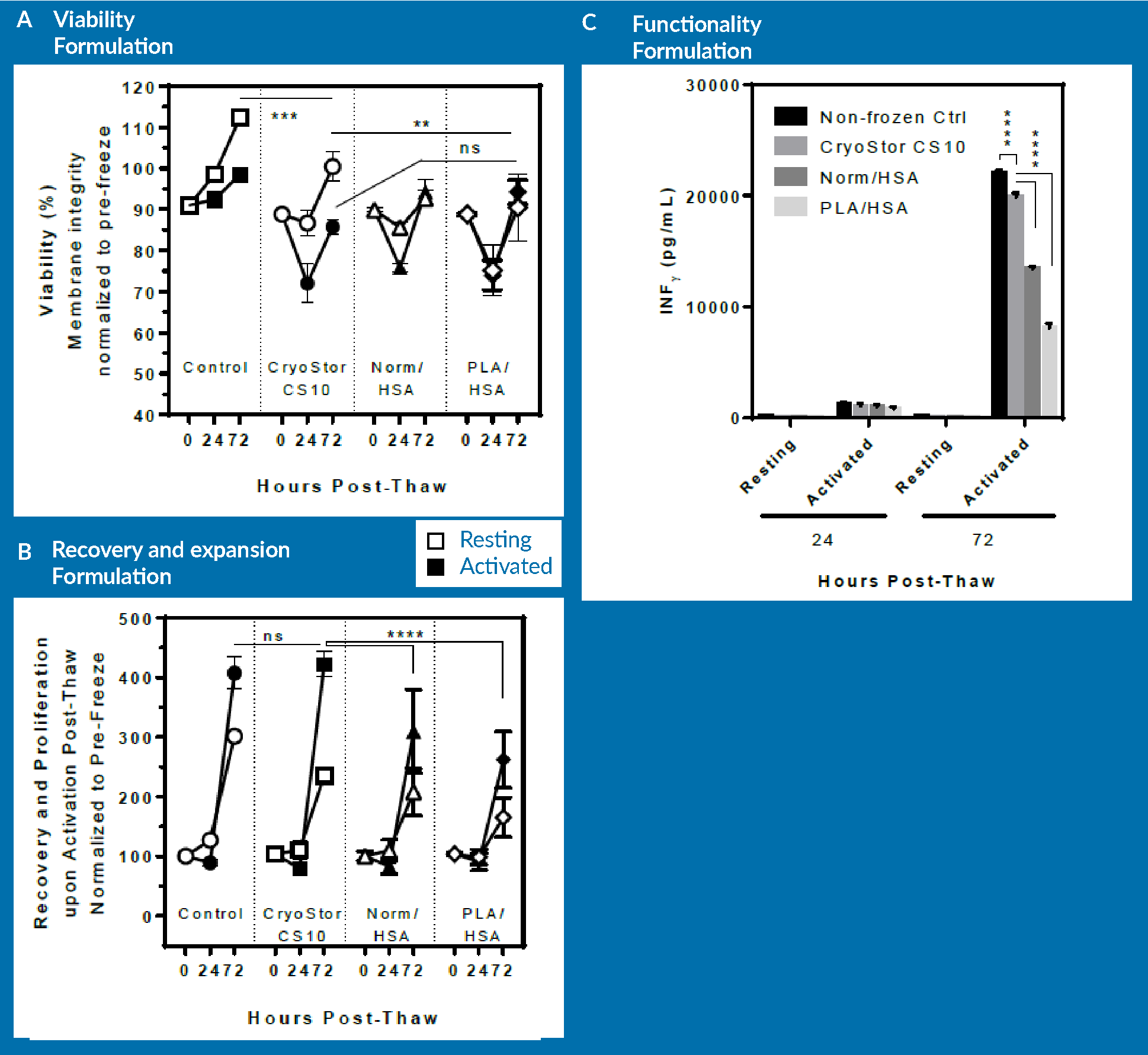
- A non-frozen control;
- Cells cryopreserved in CryoStor CS10;
- Cells cryopreserved in Normosol/HSA with 10% DMSO; and
- Cells cryopreserved in PlasmaLyte/HSA with 10% DMSO.
As observed in panel A, there is no significant difference in terms of the viability of cells that were frozen in the different formulations. The viability of the non-frozen control group rises above 100% because the viabilities were normalized to pre-freeze viability. After 3 days of post-thaw culture, cells cryopreserved in CS10 had a higher viability than the other two groups. However, when activated, all three groups behaved similarly, and exhibited a loss of viability on day 1 followed by increasing viability on day 3. However, as observed in panel B, there was a significant difference between non-frozen control/CryoStor CS10 and Normosol/PlasmaLyte in terms of expansion potential of the cells. At day 3 post-thaw, cells preserved in CryoStor CS10 proliferated at a similar rate to the non-frozen control. However, the Normosol and PlasmaLyte groups showed significantly lower expansion rate.
In terms of functionality, the cells preserved in Normosol and PlasmaLyte groups were inferior to those cryopreserved in CryoStor. However, CryoStor was still statistically significantly lower compared to non-frozen control.
In brief, the removal of serum and incorporation of intracellular-like media resulted in improved recovery and functionality of human CD3 cells upon thaw. However, it is important to note that the differences could only be observed upon follow-up with functional tests. These functional tests are essentially the expansion and secretion of cytokines. Such differences may not be clearly resolved if only relying on immediate or 24 hour post-thaw assessment.
For the purpose of exploring optimization of the DMSO concentration, the cells that were cryopreserved in CryoStor CS5 were examined against those in CryoStor CS10. Again, Figure 5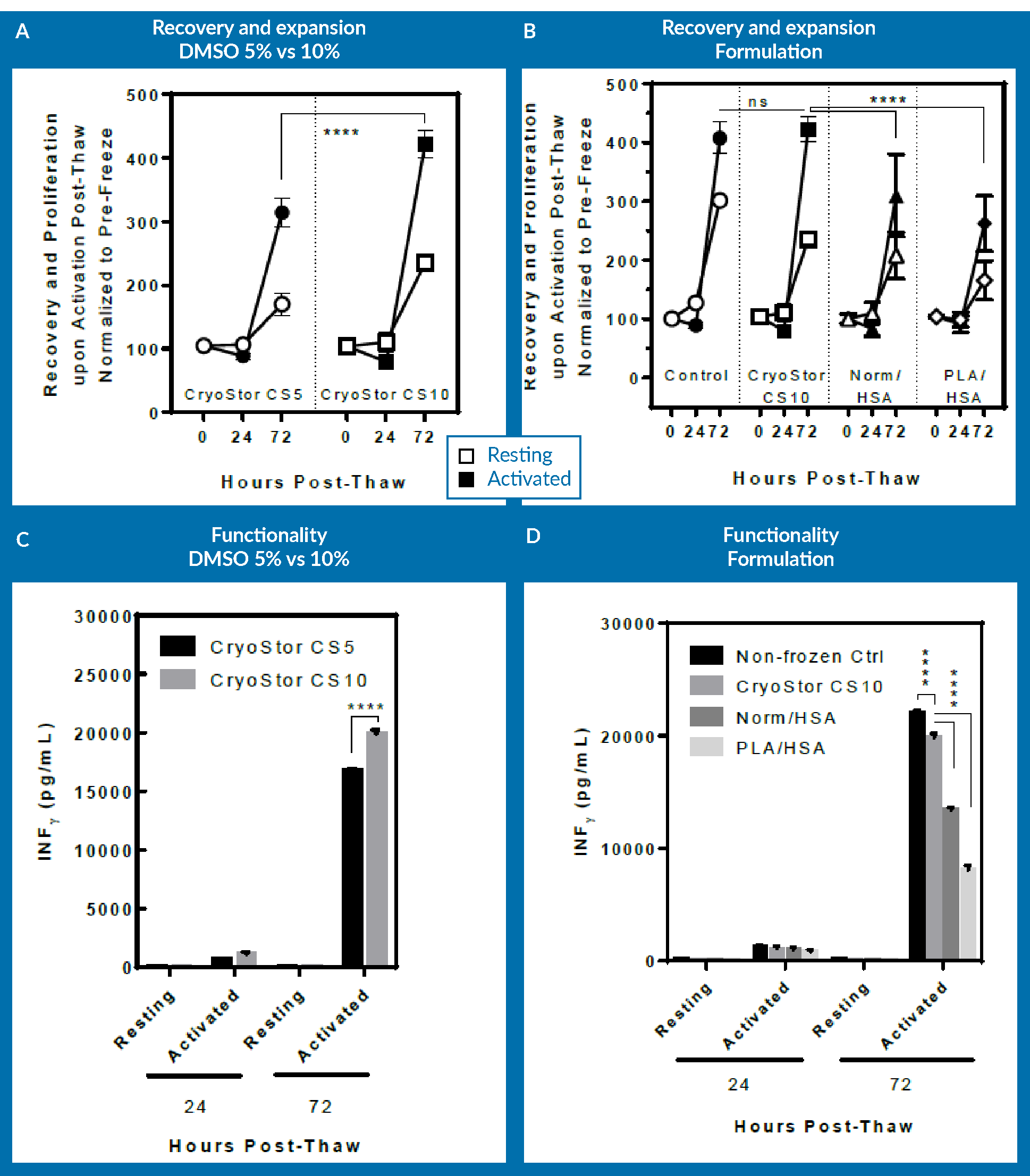
One can appreciate that CS10 did better than CS5 in increasing the DMSO concentration, and this actually resulted in an improvement in the process. However, incorporating the results from Normosol with 10% DMSO as well as PlasmaLyte-A and 10% DMSO in the analysis for comparison, at 72 hours post-thaw, CryoStor CS5 has the same recovery and expansion potential, as well as higher functionality as assessed by INF-γ secretion on day 3. This shows that changing the extracellular media to an intracellular-like media essentially allows one to decrease the necessary concentration of DMSO. Again, it is noticeable that viability immediately post-thaw did not provide an appropriate means for assessing the cells and comparing the different formulations.
A further point to mention here is regarding the excipient use model of cryopreservation media. Regarding final DMOS concentration in the product, it is a matter of risk versus benefit assessment for each group to optimize the DMSO concentration in the final product to enhance critical quality attributes. If an increase of 5% in DMSO significantly increased the potency, it would make it a lot easier to justify the use of higher DMSO concentration in your final product. However, if that increase only slightly improved the potency, then one might reasonably decide to reduce the DMSO concentration that will be administered into patient and forfeit the miniscule improvement in the CQAs of the product.
The final goal of this study was to examine whether a post-thaw delay in processing or in administering the thawed dose to patients would have any beneficial or detrimental impact on the process or product. Therefore, T cells were cryopreserved in CryoStor CS5 in a similar manner, but were thawed and activated in 3 different ways: Group 1 was thawed, processed, and activated immediately upon thaw (control/immediate process group). Group 2 were thawed, but were left to rest on bench top for 60 minutes before further processing (resuspension in media and activation step). This was to assess the post-thaw stability of the cells in the thawed cryopreservation media. Group 3 were thawed and resuspended in media and rested in the incubator for 24 hours before activation step. This was to represent an additional day in the manufacturing process to allow resting cells before activation. Activation step was conducted by adding immunoCult from StemCell Technologies, per manufacturer’s recommended protocol.
The three groups were examined for viability (Figure 6A), recovery and proliferation (Figure 6B), and INF-γ secretion (Figure 6C) at immediately, 24 hours and 72 hours post-thaw.
As seen in Figure 6A, there is no significant difference in viability across all groups at respective time-points. However, the immediately processed sample shows a significantly higher expansion potential at day 3 post-thaw compared to both the delayed sample and also the sample allowed to rest for 24 hours prior to cell activation.
It is important to mention here that the cells that were allowed to rest for 24 hours were actually activated at 24 hours post-thaw. In order to be consistent, this group was cultured up to 96 hours post-thaw, the data for which is not included in Figure 6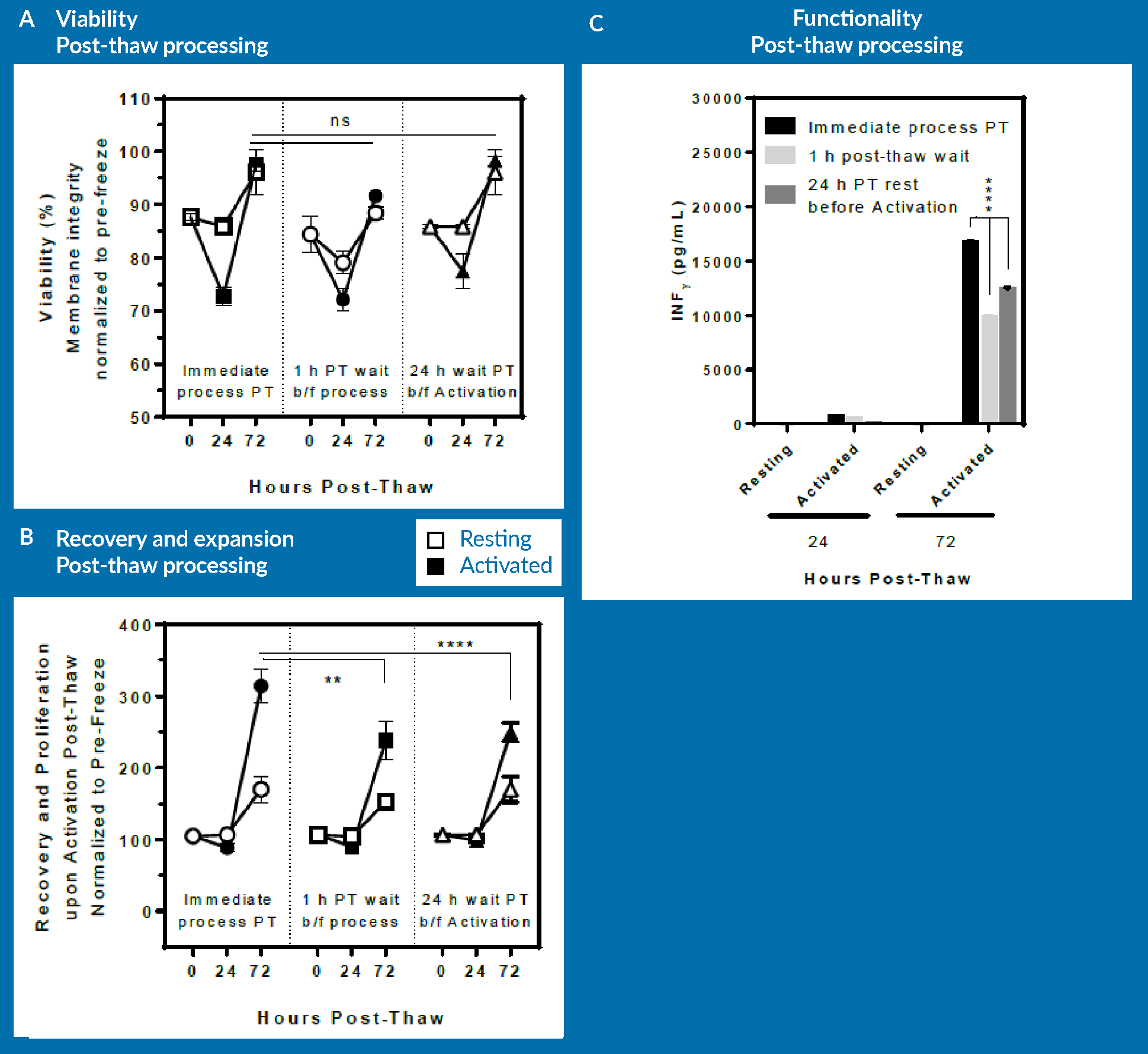
The lesson from this experiment is that a delay post-thaw translates into measurable detrimental effect on expansion potential. However, if the cells are allowed to rest in culture 24 hours prior to activation, expansion potential following activation remains unaltered. This means that a rest period post-thaw and prior to activation is not necessary for T cells. Of course, one should keep in mind that these results are obtained using one healthy donor material, and they may be different if repeated with different donor cells or with patient material. The conclusion from this is that from a manufacturing standpoint, it would be best to immediately activate upon thaw: this approach leads to the same expansion potential while essentially saving 24 hours of bioprocessing time.
In Figure 6C, a similar pattern in terms of functionality of T cells can be observed, as assessed by INF-γ secretion. The 24-hour rested group was statistically lower than the immediate post-thaw process group, but again, that was due to the sample being activated only for 48 hours rather than 72 hours for the other 2 groups.
In brief, immediate processing followed by immediate activation post-thaw appears to be advantageous compared to scenarios that allow recovery post-thaw. Further analysis would have to be conducted to identify the most cost-effective manufacturing approach based upon these results, or indeed, these experiments could simply be repeated in any lab and with any process to identify what is the most cost-effective approach for a given product.
To summarize the results of this first case study, some risk-based best practices may force changes to the process, including elimination of serum and protein. Optimization of cryopreservation process including formulation can facilitate incorporation of such risk-based best practices. Incorporation of a well-defined protein- and serum-free intracellular-like media compensated for the elimination of serum in traditional ‘home-brew’ cryopreservation media that contained higher DMSO content.
Identifying critical process parameters of cryopreservation using Jurkat cells.
The second case study identifies some of the process parameters of the cryopreservation step that are perhaps more obscure. The Jurkat T cell model was used to study a variety of different process parameters, but for the purposes of this presentation, the focus is solely on one particular aspect of the freezing profile, and a particular aspect of how packaging impacts on CQAs.
The Jurkat T cells had been frozen in CryoStor CS5. After storage in liquid nitrogen and thawing, the cells were followed up for 48 hours, looking at viability and count post-thaw. Count or proliferation post-thaw would be the ultimate functional test for these Jurkat T cells.
The impact of proper nucleation, which is a small but very important part of the freezing profile, was recorded. For container impact, the indirect impact of container choice on the freezing rate was assessed, as were the CQAs of the products as determined by viability and proliferation rate.
There is a very popular school classroom experiment where one freezes a supercooled water bottle. A supercooled condition is an unstable thermodynamic state when the temperature is below the freezing point but ice crystals are yet to form. Disruption of this equilibrium results in the system going back to the minimal energy state, i.e., ice crystals. The intention with this experiment was to do the same with the freezing profile. In a standard freezing profile, a nucleation step is designed specifically to disrupt this pseudo-equilibrium state in vials or bags and initiate nucleation. If nucleation is allowed to occur spontaneously, without activation, one starts to see significant variability in the product. In a freezing process without active nucleation, cells start to freeze randomly at different temperatures below the freezing point. However, if active nucleation is incorporated into the freezing process, all of the vials freeze uniformly at the same time. This can eliminate a significant source of variability in final product CQAs.
A sample of cells was firstly frozen down and nucleated properly at around -10°C. A second sample was also allowed to freeze but the cells were not nucleated, meaning the equilibrium was not disrupted and nucleation was allowed to happen spontaneously or stochastically.
It is interesting to note that at 24 hours post-thaw, both groups come out with identical viabilities, or viable recoveries. If one relies solely on viability or viable recovery, there was no discernible difference between the two approaches. The difference is only visible at 24 hours post-thaw, when one begins to see a detrimental effect on the proliferation capacity of the non-nucleated group. This is a very important factor that contributes to significant variability in cell product. It is suggested that process development scientists should always ensure that proper control is maintained over this step and that rigorous process development around this step is carried out to minimize the potentially damaging impact on viability caused by spontaneous or stochastic nucleation.
Turning to the impact of the freezing container, this particular experiment was born out of a conversation with a customer some years ago. The customer in question mentioned that physicians or nurses very much prefer to have cryopreserved product in a vial due to physicians’ preference in working with vials and syringes rather than bags. This study was designed to ascertain whether the geometry of the vial could impact the outcome of the freezing process.
The freezing process was simulated in the largest vial, shown on the left in Figure 7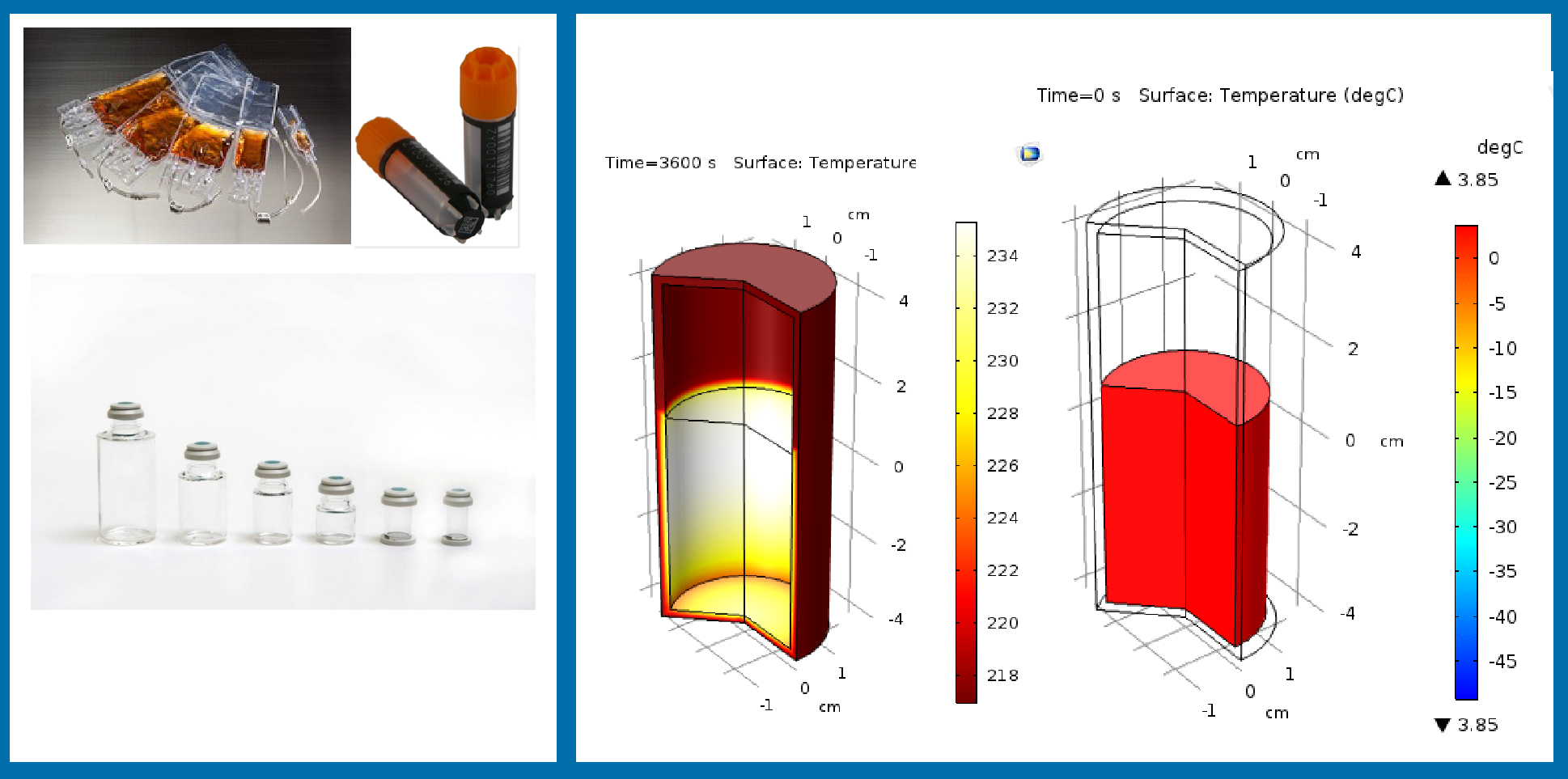
This simulation demonstrates that as the temperature drops on the outside at a common rate of 1°C/minute, the gradient of temperature between inside and outside the vial can reach to as high as 35C. This translates into significant variation in freezing rate that cells experience during freezing, with a large portion of the vial contents freezing at 0.5C/min or slower.
To test whether slower freezing rates can adversely impact the cryopreservation process outcome, Jurkat cells were cryopreserved at 0.5C/min or 1C/min. The results suggested that a freezing rate of 0.5°C/minute translates into significant loss of proliferation capacity for the Jurkat cells: at 24 hours post-thaw, there is essentially no proliferation in the population of Jurkat cells that had frozen at 0.5°C/minute.
This demonstrates an important process parameter that process development scientists should be aware of, as well as the capacity of the geometry and material of the storage container to significantly impact the CQAs of the product.
In summary, in-depth knowledge of the cryopreservation process is required to identify the critical process parameters that impact the quality attributes of the product. Robust assays and long-term (>24-hour) post-thaw follow-up is necessary to fully evaluate and understand the impact of process parameters including media, and changes to various process parameters that may impact product CQAs.
Again, it is important to stress that immediate post-thaw viability, especially after cryopreservation and particularly as measured by membrane integrity, is not an accurate representation of how well that cryopreservation process was conducted. It is strongly recommended that development scientists identify other assays beyond membrane integrity or conduct long-term follow-up testing after cryopreservation and thaw to identify how that cryopreservation process was conducted and how it impacted the cells.
Conclusion
In conclusion, incorporation of Biopreservation Best Practices is a multi-faceted approach that addresses numerous commercial, quality/regulatory and process development concerns. Proper knowledge and understanding of cellular response to cold and freezing is necessary for identification of critical process parameters that impact the CQAs of the product.
From the Q&A
Q How often does optimization of cryopreservation involve only substitution of the freeze media versus optimization of process development steps outside of the media?
In our experience, we have realized that optimizing the freezing media improves the results significantly. Much of the time, this meets a developer’s minimum requirements. With certain cell types, you may need to look at other process parameters such as the freezing profile, which is a key element of optimizing the process. However, if you are using an optimized media, it can alleviate some of these requirements.
QDo any of the products discussed here that were used by BioLife Solutions have FDA approval?
The FDA is concerned with medical devices and drugs and none of BioLife Solutions’ products are categorized as such. BioLife Solutions’ products are typically used as ancillary material or excipient. Ancillary products assist in the production of the end product but are completely removed from that product prior to patient administration. BioLife Solutions’ product can be used as excipient, which means that the cells and media combined are treated as one product that is to be approved by the FDA.
QWhat are the pros and cons of washing the cell product post-thaw to remove the cryoprotectant?
You may have elements of the cryomedia that must be removed from the product as they do not meet the requirements for patient administration. However, the washing step means an additional manufacturing step. The product of the washing step is in liquid form, which tend to have shorter shelf-life. It is important to think about transporting the product more efficiently. You will also need expertise at the patient’s bedside to administer the product to the patient. This adds time and cost to the manufacturing process and is the reason why most companies are not removing the cryoprotectant post-thaw and are instead aiming for approval of it as an excipient.
QAre there ways to improve the stability of apheresis material between collection and processing?
There are two modes of transporting the material: fresh and cryopreserved. In the fresh form, you may seek to use an optimized media for the transport and delivery of the cells. If you are transporting fresh cells at 2°C -8°C degrees, the stress of cold can impact the quality of the product. This is why using an optimized media, designed to mitigate the cold stress on the cells, will result in increased durability of the starting material.
Ask the Scientists
Additional questions can be addressed through ‘Ask the Scientists’ on the Cell and Gene Therapy Insights website.
In partnership with BioLife Solutions, Cell & Gene Therapy Insights is giving you the chance to have your biopreservation questions answered throughout 2019.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest.
Funding declaration:The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2019 Abazari A. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source:Webinar Transcript
Webinar streamed: June 18 2019; Publication date: October 25 2019.
Affiliation
Alireza Abazari
Scientific Applications Director,
BioLife Solutions

