Expression systems for viral vector production: advantages of the Sf9 Baculovirus system and simple solutions to address its specific analytical challenges
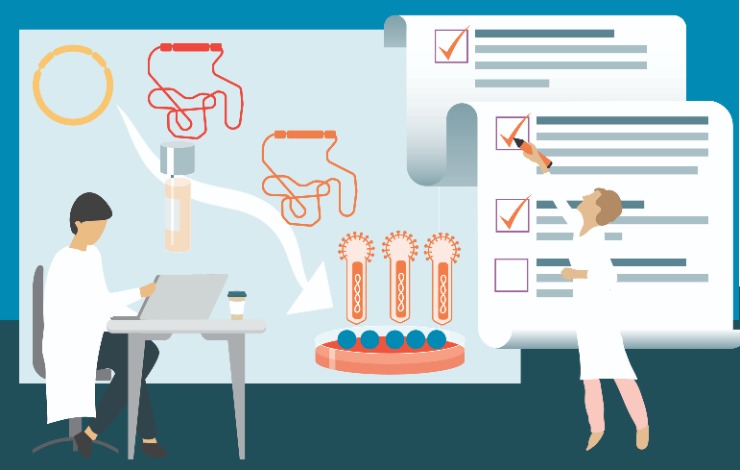
The promise of gene therapy depends greatly on the ability to scale processes from the bench to a highly regulated cGMP manufacturing environment while ensuring the purity, quality, and safety of the final product. At larger scales in particular, the baculovirus expression system in Spodoptera frugiperda Sf9, Sf21 and other insect host cells holds various advantages over other common mammalian expression systems for viral vector production. However, the baculovirus system does have a few host system-specific analytical challenges. Therefore, highly reliable, sensitive analytical assays are critical to demonstrate removal of product or process-related impurities during development, scale-up, and lot-release.
In this webinar, we will compare different viral vector production platforms with a focus on the advantages and specific analytical challenges of the baculovirus expression system in Sf9 cells. You will learn about process analytics and lot-release assay requirements for viral vector-based advanced therapy medicinal products (ATMP). We will share simple solutions for the quantitation of residual DNA and potential Sf-Rhabdovirus contaminants applicable to this expression system at any stage in the development process, in preparation for manufacturing success.
- An overview of the Sf9 Baculovirus expression system for gene therapy products
- A review of the regulatory guidance for gene therapy products and those relevant to the Sf9 / Baculovirus system in particular
- Detailed data and performance specification of an accurate, highly sensitive solution for Sf-Rhabdovirus quantitation in Sf cell lines
- Duplex real-time PCR solution for the simultaneous accurate quantitation of Sf9 host cell and baculovirus residual DNA
You might also like

Same-section spatial multiomics: a platform for detailed analysis of the solid tumor TME
Precision in production: optimizing monitoring and quality control for high-value plasmids
Advancing cell therapy: innovations in polymer-based encapsulation and delivery

Pure and simple: understanding LNP analytics for better mRNA-based drugs




