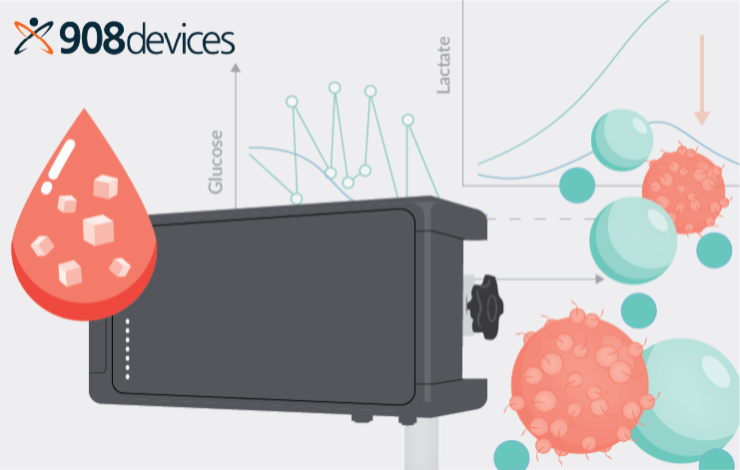
Improving cell therapy manufacturing with on-line monitoring of critical process parameters during cell expansion
Live30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format.
Automated manufacturing systems offer significant benefits to cell therapy manufacturing, such as reducing manual intervention, improving consistency, and lowering overall costs. However, fully transitioning from manual protocols to automated processing necessitates the integration of real-time process parameters in development and cGMP manufacturing.
Glucose and lactate concentrations can serve as leading indicators of the health and growth kinetics of cells. When considered alongside the perfusion rate, these metabolic indicators can help optimize media usage and determine the optimal harvest time. The synergy between automated cell expansion systems like Quantum Flex and MAVEN helps enhance process understanding and facilitates dynamic adjustments to process conditions.
This webinar will explore how automated monitoring and control of glucose and lactate levels can significantly improve the cell therapy process.
- Explore methodologies to achieve greater robustness and lower cost of goods in cell therapy manufacturing
- Understand the benefits and considerations in real-time monitoring of key process parameters such as glucose and lactate
- Learn how to enable the creation and maintenance of optimal cell expansion conditions
You might also like

Developing off-the-shelf CAR-NK cells targeting CD70: updates from preclinical and early clinical studies

Characterization and release testing for AAV therapies: empty/partial/full capsid analysis and impact on potency
Development of a non-viral gene delivery platform for CAR-T manufacturing
Supporting efficacy and scaling with a next-generation T cell AOF formulation medium